LIPERCOSYL Capsules, hard Ref.[107849] Active ingredients: Atorvastatin Atorvastatin and Perindopril Perindopril
Source: Health Products Regulatory Authority (IE) Revision Year: 2022 Publisher: Les Laboratoires Servier, 50, rue Carnot, 92284 Suresnes Cedex, France
4.3. Contraindications
- Hypersensitivity to the active substances or to any other ACE (Angiotensin Converting Enzyme) inhibitor or statin or to any of the excipients of this medicinal product listed in section 6.1;
- Active liver disease or unexplained persistent elevations of serum transaminases exceeding 3 times the upper limit of normal;
- During pregnancy, while breast-feeding and in women of child-bearing potential not using appropriate contraceptive measures (see section 4.6);
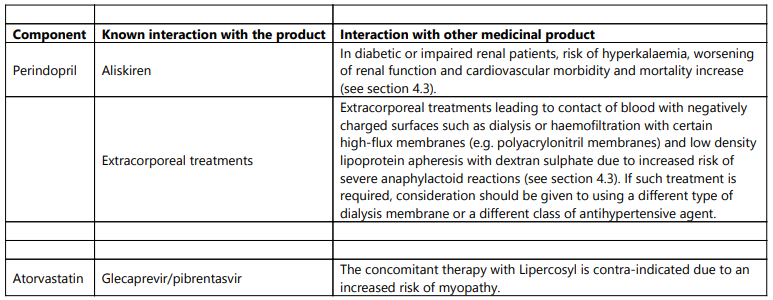
- Concomitant use with the hepatitis C antivirals glecaprevir/pibrentasvir;
- History of angioedema associated with previous ACE inhibitor therapy;
- Hereditary or idiopathic angioedema;
- Concomitant use with aliskiren-containing products in patients with diabetes mellitus or renal impairment (GFR <60 mL/min/1.73 m²) (see sections 4.5 and 5.1);
- Concomitant use with sacubitril/valsartan therapy. Lipercosyl must not be initiated earlier than 36 hours after the last dose of sacubitril/valsartan (see sections 4.4 and 4.5);
- Extracorporeal treatments leading to contact of blood with negatively charged surfaces (see section 4.5);
- Significant bilateral renal artery stenosis or stenosis of the artery to a single functioning kidney (see section 4.4).
4.4. Special warnings and precautions for use
Special warnings and precautions related to atorvastatin and perindopril are applicable to Lipercosyl
Liver effects
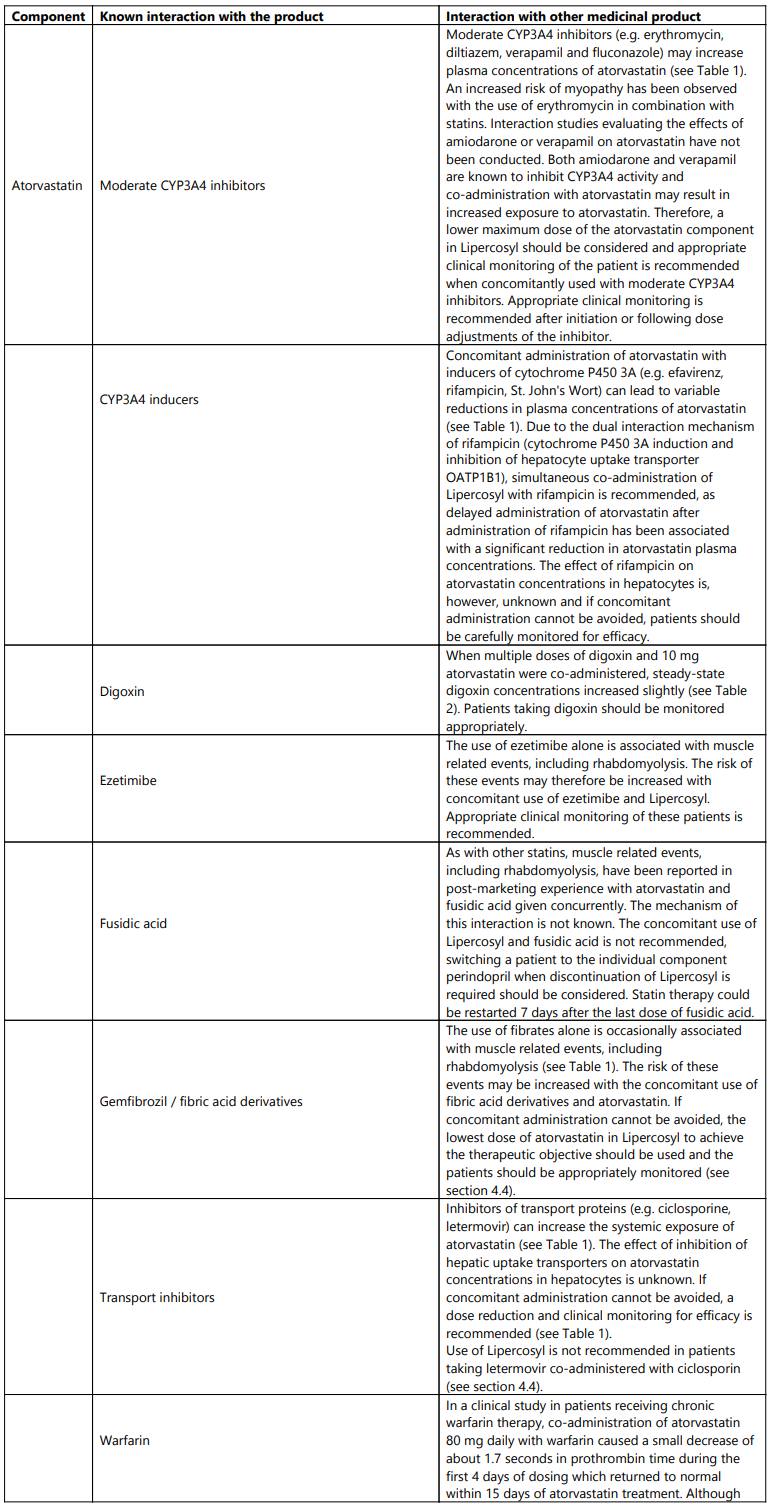
Due to the atorvastatin component in Lipercosyl, liver function tests should be performed periodically. Patients who develop any signs or symptoms suggestive of hepatic dysfunction should have liver function tests performed. Patients who develop increased transaminase levels should be monitored until the abnormality (ies) resolve. Should an increase in transaminases of greater than 3 times the upper limit of normal (ULN) persist, reduction of atorvastatin dose using the individual components or withdrawal of atorvastatin is recommended (see section 4.8).
Rarely, ACE inhibitors such as perindopril have been associated with a syndrome that starts with cholestatic jaundice and progresses to fulminant hepatic necrosis and (sometimes) death. The mechanism of this syndrome is not understood. Patients receiving Lipercosyl who develop jaundice or marked elevations of hepatic enzymes should discontinue the product and receive appropriate medical follow-up (see section 4.8).
Taking into account the effect of atorvastatin and perindopril, Lipercosyl is contra-indicated in patients with active liver disease or unexplained persistent elevations of serum transaminases exceeding 3 times the upper limit of normal (see section 4.3). Lipercosyl should be used with caution in patients with hepatic impairment and in patients who consume substantial quantities of alcohol and/or have a history of liver disease. If a change of posology is required, titration should be done with the individual components.
Skeletal muscle effects
Atorvastatin, like other HMG-CoA reductase inhibitors, may in rare occasions affect the skeletal muscle and cause myalgia, myositis, and myopathy that may progress to rhabdomyolysis, a potentially life-threatening condition characterised by markedly elevated creatine kinase (CK) levels (>10 times ULN), myoglobinaemia and myoglobinuria which may lead to renal failure.
In situations where pre-disposing factors for rhabdomyolysis have been identified before treatment initiation i.e.:
- Renal impairment
- Hypothyroidism
- Personal or familial history of hereditary muscular disorders
- Previous history of muscular toxicity with a statin or fibrate
- Previous history of liver disease and/or where substantial quantities of alcohol are consumed
- In elderly (age >70 years), the need for CK measurement should be considered, taking account of the presence of other predisposing factors for rhabdomyolysis
- When an increase in plasma levels may occur, such as interactions (see section 4.5) and special populations including genetic subpopulations (see section 5.2) the risk of treatment should be considered in relation to possible benefit, and clinical monitoring is recommended. If CK levels are significantly elevated (>5 times ULN) at baseline, treatment should not be started.
Creatine kinase measurement
Creatine kinase (CK) should not be measured following strenuous exercise or in the presence of any plausible alternative cause of CK increase as this makes value interpretation difficult. If CK levels are significantly elevated at baseline (> 5 times ULN), levels should be measured again within 5 to 7 days later to confirm the results.
Whilst on treatment
- Patients must be asked to promptly report muscle pain, cramps, or weakness especially if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuing Lipercosyl.
- If such symptoms occur whilst a patient is receiving treatment with Lipercosyl, their CK levels should be measured. If these levels are found to be significantly elevated (>5 times ULN), treatment should be stopped.
- If muscular symptoms are severe and cause daily discomfort, even if the CK levels are elevated to ≤5 x ULN, treatment discontinuation should be considered.
- If symptoms resolve and CK levels return to normal, then re-introduction of atorvastatin or introduction of an alternative statin may be considered at the lowest dose and with close monitoring.
- Lipercosyl must be discontinued immediately if clinically significant elevation of CK levels (>10 x ULN) occur, or if rhabdomyolysis is diagnosed or suspected. Concomitant treatment with other medicinal products Due to atorvastatin component, risk of rhabdomyolysis is increased when Lipercosyl is administered concomitantly with certain medicinal products that may increase the plasma concentration of atorvastatin such as potent inhibitors of CYP3A4 or transport proteins (e.g. ciclosporine, telithromycin, clarithromycin, delavirdine, stiripentol, ketoconazole, voriconazole, itraconazole, posaconazole, letermovir and HIV protease inhibitors including ritonavir, lopinavir, atazanavir, indinavir, darunavir, tipranavir/ritonavir, etc). The risk of myopathy may also be increased with the concomitant use of gemfibrozil and other fibric acid derivates, antivirals for the treatment of hepatitis C (HCV) (boceprevir, telaprevir, elbasvir/grazoprevir), erythromycin, niacin or ezetimibe. If possible, alternative (non-interacting) therapies should be considered instead of these medicinal products.
In cases where co-administration of these medicinal products with Lipercosyl is necessary, the benefit and the risk of concurrent treatment should be carefully considered. When patients are receiving medicinal products that increase the plasma concentration of atorvastatin, a lower maximum dose of atorvastatin is recommended, hence down-titration with the individual components should be considered. In addition, in the case of potent CYP3A4 inhibitors, a lower starting dose of atorvastatin should be considered and appropriate clinical monitoring of these patients is recommended (see section 4.5).
Atorvastatin must not be co-administered with systemic formulations of fusidic acid or within 7 days of stopping fusidic acid treatment. In patients where the use of systemic fusidic acid is considered essential, statin treatment should be discontinued throughout the duration of fusidic acid treatment. There have been reports of rhabdomyolysis (including some fatalities) in patients receiving fusidic acid and statins in combination (see section 4.5). The patient should be advised to seek medical advice immediately if they experience any symptoms of muscle weakness, pain or tenderness.
Statin therapy may be re-introduced seven days after the last dose of fusidic acid.
In exceptional circumstances, where prolonged systemic fusidic acid is needed, e.g., for the treatment of severe infections, the need for co-administration of Lipercosyl and fusidic acid should only be considered on a case by case basis and under close medical supervision.
Immune-mediated necrotizing myopathy
There have been very rare reports of an immune-mediated necrotizing myopathy (IMNM) during or after treatment with some statins. IMNM is clinically characterised by persistent proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment.
Interstitial lung disease
Exceptional cases of interstitial lung disease have been reported with some statins, especially with long term therapy (see section 4.8). Presenting features can include dyspnoea, non-productive cough and deterioration in general health (fatigue, weight loss and fever). If it is suspected a patient has developed interstitial lung disease, Lipercosyl therapy should be discontinued and switching to therapy with only perindopril should be considered.
Diabetes Mellitus
Some evidence suggests that statins as a class raise blood glucose and in some patients, at high risk of future diabetes, may produce a level of hyperglycaemia where formal diabetes care is appropriate. This risk, however, is outweighed by the reduction in vascular risk with statins and therefore should not be a reason for stopping Lipercosyl treatment. Patients at risk (fasting glucose 5.6 to 6.9 mmol/L, BMI > 30 kg/m², raised triglycerides, hypertension) should be monitored both clinically and biochemically according to national guidelines when treated with Lipercosyl. In diabetic patients treated with oral antidiabetic agents or insulin, glycaemic control should be closely monitored during the first month of treatment with medicines containing an ACE inhibitor, such as Lipercosyl (see section 4.5).
Hypotension
ACE inhibitors, such as perindopril, may cause a fall in blood pressure. Symptomatic hypotension is seen rarely in uncomplicated hypertensive patients and is more likely to occur in patients who have been volume-depleted e.g. by diuretic therapy, dietary salt restriction, dialysis, diarrhoea or vomiting, or who have severe renin-dependent hypertension (see sections 4.5 and 4.8). In patients with symptomatic heart failure, with or without associated renal insufficiency, symptomatic hypotension has been observed. This is most likely to occur in those patients with more severe degrees of heart failure, as reflected by the use of high doses of loop diuretics, hyponatraemia or functional renal impairment. In patients at increased risk of symptomatic hypotension, initiation of therapy and dose adjustment should be closely monitored (see sections 4.2 and 4.8). Similar considerations apply to patients who suffer from ischaemic heart or cerebrovascular disease in whom an excessive fall in blood pressure could result in a myocardial infarction or cerebrovascular accident.
If hypotension occurs, the patient should be placed in the supine position and, if necessary, should receive an intravenous infusion of sodium chloride 9 mg/mL (0.9 %) solution. A transient hypotensive response is not a contraindication to further doses, which can be given usually without difficulty once the blood pressure has increased after volume expansion.
In some patients with congestive heart failure who have normal or low blood pressure, additional lowering of systemic blood pressure may occur with perindopril. This effect is anticipated and is usually not a reason to discontinue treatment. If hypotension becomes symptomatic, a reduction of dose using the individual components or discontinuation of treatment with Lipercosyl may be necessary.
Aortic and mitral valve stenosis/ hypertrophic cardiomyopathy
As with other medicines containing ACE inhibitors such as perindopril, Lipercosyl should be given with caution to patients with mitral valve stenosis and obstruction in the outflow of the left ventricle such as aortic stenosis or hypertrophic cardiomyopathy.
Kidney transplantation
There is no experience regarding the administration of perindopril or Lipercosyl in patients with a recent kidney transplantation.
Renovascular hypertension
There is an increased risk of hypotension and renal insufficiency when patient with bilateral renal artery stenosis or stenosis of the artery to a single functioning kidney are treated with ACE inhibitors (see section 4.3). Treatment with diuretics may be a contributory factor. Loss of renal function may occur with only minor changes in serum creatinine even in patients with unilateral renal artery stenosis.
Renal impairment
Lipercosyl can be administered in patients with creatinine clearance >60 mL/min, and is not suitable for patients with moderate renal impairment (creatinine clearance between 30 and 60 mL/min) or with severe renal impairment (creatinine clearance <30 mL/min). In these patients, an individual dose titration with the monocomponents is recommended. Routine monitoring of potassium and creatinine are part of normal medical practice for patients with renal impairment (see section 4.8).
In some patients with bilateral renal artery stenosis or stenosis of the artery to a solitary kidney, who have been treated with ACE inhibitors, increases in blood urea and serum creatinine, usually reversible upon discontinuation of therapy, have been seen. This is especially likely in patients with renal insufficiency. If renovascular hypertension is also present there is an increased risk of severe hypotension and renal insufficiency.
Some hypertensive patients with no apparent pre-existing renal vascular disease have developed increases in blood urea and serum creatinine, usually minor and transient, especially when perindopril has been given concomitantly with a diuretic. This is more likely to occur in patients with pre-existing renal impairment. Dosage reduction using the individual components and/or discontinuation of the diuretic and/or Lipercosyl may be required.
The effect of the combination Lipercosyl has not been tested in patients with renal impairment. Lipercosyl doses should respect the dosing recommendations of the individual components taken separately.
Haemodialysis patients
Anaphylactoid reactions have been reported in patients dialysed with high flux membranes, and treated concomitantly with an ACE inhibitor. In these patients consideration should be given to using a different type of dialysis membrane or different class of antihypertensive agent.
Hypersensitivity/Angioedema
Angioedema of the face, extremities, lips, mucous membranes, tongue, glottis and/or larynx has been reported rarely in patients treated with ACE inhibitors, including perindopril (see section 4.8). This may occur at any time during therapy. In such cases, Lipercosyl should promptly be discontinued and appropriate monitoring should be initiated and continued until complete resolution of symptoms has occurred. In those instances where swelling was confined to the face and lips the condition generally resolved without treatment, although antihistamines have been useful in relieving symptoms.
Angioedema associated with laryngeal oedema may be fatal. Where there is involvement of the tongue, glottis or larynx, likely to cause airway obstruction, emergency therapy should be administered promptly. This may include the administration of adrenaline and/or the maintenance of a patent airway. The patient should be under close medical supervision until complete and sustained resolution of symptoms has occurred.
Patients with a history of angioedema unrelated to ACE inhibitor therapy may be at increased risk of angioedema while receiving Lipercosyl (see section 4.3).
Intestinal angioedema has been reported rarely in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan, or ultrasound or at surgery and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should be included in the differential diagnosis of patients treated with Lipercosyl presenting with abdominal pain.
The combination of perindopril with sacubitril/valsartan is contraindicated due to the increased risk of angioedema (see section 4.3). Sacubitril/valsartan must not be initiated until 36 hours after taking the last dose of perindopril therapy. If treatment with sacubitril/valsartan is stopped, perindopril therapy must not be initiated until 36 hours after the last dose of sacubitril/valsartan (see sections 4.3 and 4.5). Concomitant use of ACE inhibitors with NEP inhibitors (e.g. racecadotril), mTOR inhibitors (e.g. sirolimus, everolimus, temsirolimus) and gliptins (e.g. linagliptin, saxagliptin, sitagliptin, vildagliptin) may lead to an increased risk of angioedema (e.g. swelling of the airways or tongue, with or without respiratory impairment) (see section 4.5). Caution should be used when starting racecadotril, mTOR inhibitors (e.g. sirolimus, everolimus, temsirolimus) and gliptins (e.g. linagliptin, saxagliptin, sitagliptin, vildagliptin) in a patient already taking an ACE inhibitor.
Anaphylactoid reactions during low‑density lipoproteins (LDL) apheresis
Rarely, patients receiving ACE inhibitors such as perindopril during low-density lipoprotein (LDL) apheresis with dextran sulfate have experienced life-threatening anaphylactoid reactions. These reactions were avoided by temporarily withholding ACE inhibitor therapy prior to each apheresis.
Anaphylactic reactions during desensitisation
Patients receiving ACE inhibitor-containing medicines, such as Lipercosyl, during desensitisation treatment (e.g. hymenoptera venom) have experienced anaphylactoid reactions. In the same patients, these reactions have been avoided when the ACE inhibitors were temporarily withheld, but they reappeared upon inadvertent rechallenge.
Neutropenia/Agranulocytosis/Thrombocytopenia/Anaemia
Neutropenia/agranulocytosis, thrombocytopenia and anaemia have been reported in patients receiving ACE inhibitors. In patients with normal renal function and no other complicating factors, neutropenia occurs rarely. Lipercosyl should be used with extreme caution in patients with collagen vascular disease, immunosuppressant therapy, treatment with allopurinol or procainamide, or a combination of these complicating factors, especially if there is pre-existing impaired renal function. Some of these patients developed serious infections, which in a few instances did not respond to intensive antibiotic therapy. If Lipercosyl is used in such patients, periodic monitoring of white blood cell counts is advised and patients should be instructed to report any sign of infection (e.g. sore throat, fever).
Race
ACE inhibitors cause a higher rate of angioedema in black patients than in non-black patients.
Lipercosyl, which contains the ACE inhibitor perindopril, may be less effective in lowering blood pressure in black people than in non-blacks, possibly because of a higher prevalence of low-renin states in the black hypertensive population.
Cough
Cough has been reported with the use of ACE inhibitors. Characteristically, the cough is non-productive, persistent and resolves after discontinuation of therapy. ACE inhibitor-induced cough should be considered as part of the differential diagnosis of cough in patients treated with Lipercosyl.
Surgery/Anaesthesia
In patients undergoing major surgery or during anaesthesia with agents that produce hypotension, Lipercosyl may block angiotensin II formation secondary to compensatory renin release. The treatment should be discontinued one day prior to the surgery. If hypotension occurs and is considered to be due to this mechanism, it can be corrected by volume expansion.
Hyperkalaemia
Elevations in serum potassium have been observed in some patients treated with ACE inhibitors, including perindopril, ACE inhibitors can cause hyperkalaemia because they inhibit the release of aldosterone. The effect is usually not significant in patients with normal renal function. Risk factors for the development of hyperkalaemia include those with renal insufficiency, worsening of renal function, age (>70 years), diabetes mellitus, intercurrent events, in particular dehydration, acute cardiac decompensation, metabolic acidosis and concomitant use of potassium-sparing diuretics (e.g. spironolactone, eplerenone, triamterene, or amiloride), potassium supplements or potassium-containing salt substitutes; or those patients taking other drugs associated with increases in serum potassium (e.g. heparin, co-trimoxazole also known as trimethoprim/sulfamethoxazole) and especially aldosterone antagonists or angiotensin-receptor blockers. The use of potassium supplements, potassium-sparing diuretics, or potassium-containing salt substitutes particularly in patients with impaired renal function may lead to a significant increase in serum potassium. Hyperkalaemia can cause serious, sometimes fatal arrhythmias. Potassium-sparing diuretics and angiotensin-receptor blockers should be used with caution in patients receiving ACE inhibitors, and serum potassium and renal function should be monitored. If concomitant use of the above-mentioned agents with Lipercosyl is deemed appropriate, they should be used with caution and with frequent monitoring of serum potassium (see section 4.5).
Combination with lithium
The combination of lithium and medicines containing perindopril, such as Lipercosyl, is not recommended (see section 4.5).
Dual blockade of the renin‑angiotensin‑aldosterone system (RAAS)
There is evidence that the concomitant use of ACE-inhibitors, angiotensin II receptor blockers or aliskiren increases the risk of hypotension, hyperkalaemia and decreased renal function (including acute renal failure). Dual blockade of RAAS through the combined use of ACE-inhibitors, angiotensin II receptor blockers or aliskiren is therefore not recommended (see sections 4.5 and 5.1).
If dual blockade therapy is considered absolutely necessary, this should only occur under specialist supervision and subject to frequent close monitoring of renal function, electrolytes and blood pressure.
ACE-inhibitors and angiotensin II receptor blockers should not be used concomitantly in patients with diabetic nephropathy.
Primary aldosteronism
Patients with primary hyperaldosteronism generally will not respond to anti-hypertensive drugs acting through inhibition of the renin-angiotensin system. Therefore, the use of this product is not recommended.
Excipients
Due to the presence of sucrose, patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take Lipercosyl.
Level of sodium
contains less than 1 mmol sodium (23 mg) per capsule, i.e. essentially ‘sodium-free’.
4.5. Interaction with other medicinal products and other forms of interaction
No drug interaction studies have been conducted with Lipercosyl and other drugs, although studies have been conducted with atorvastatin and perindopril separately. The results of these studies are provided below.
Clinical trial data has shown that dual blockade of the renin-angiotensin-aldosterone-system (RAAS) through the combined use of ACE-inhibitors, angiotensin II receptor blockers or aliskiren is associated with a higher frequency of adverse events such as hypotension, hyperkalaemia and decreased renal function (including acute renal failure) compared to the use of a single RAAS-acting agent (see sections 4.3, 4.4 and 5.1).
Drugs increasing the risk of angioedema
Concomitant use of ACE inhibitors with sacubitril/valsartan is contraindicated as this increases the risk of angioedema (see section 4.3 and 4.4). Sacubitril/valsartan must not be started until 36 hours after taking the last dose of perindopril therapy. Perindopril therapy must not be started until 36 hours after the last dose of sacubitril/valsartan (see sections 4.3 and 4.4). Concomitant use of ACE inhibitors with racecadotril, mTOR inhibitors (e.g. sirolimus, everolimus, temsirolimus) and gliptins (e.g. linagliptin, saxagliptin, sitagliptin, vildagliptin) may lead to an increased risk for angioedema (see section 4.4).
Drugs inducing hyperkalaemia
Although serum potassium usually remains within normal limits, hyperkalaemia may occur in some patients treated with Lipercosyl. Some drugs or therapeutic classes may increase the occurrence of hyperkalaemia: aliskiren, potassium salts, potassium-sparing diuretics (e.g. spironolactone, triamterene or amiloride), ACE inhibitors, angiotensin-II receptors antagonists, NSAIDs, heparins, immunosuppressant agents such as ciclosporine or tacrolimus, trimethoprim and cotrimoxazole (trimethoprim/sulfamethoxazole), as trimethoprim is known to act as a potassium-sparing diuretic like amiloride. The combination of these drugs increases the risk of hyperkalaemia. Therefore, the combination of Lipercosyl with the above-mentioned drugs is not recommended. If concomitant use is indicated, they should be used with caution and with frequent monitoring of serum potassium.
Concomitant use contraindicated (see section 4.3)
Concomitant use not recommended (see section 4.4)
Concomitant use which requires special care
Concomitant use to be taken into consideration
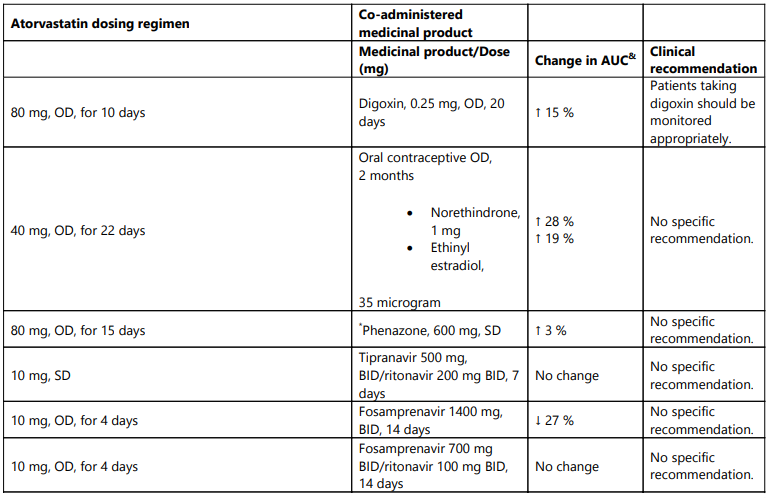
Table 1. Effect of co-administered medicinal product on the pharmacokinetics of atorvastatin:
OD = once daily, SD = single dose, BID = twice daily, QID = Four times daily, TID = three times daily
Increase is indicated as “↑”, decrease as "↓"
& Data given as x-fold change represent a simple ratio between co-administration and atorvastatin alone (i.e., 1-fold = no change). Data given as % change represent % difference relative to atorvastatin alone (i.e., 0 % = no change).
# See sections 4.4 and 4.5 for clinical significance.
* Contains one or more components that inhibit CYP3A4 and can increase plasma concentrations of medicinal products metabolized by CYP3A4. Intake of one 240 mL glass of grapefruit juice also resulted in a decreased AUC of 20.4 % for theactive orthohydroxy metabolite. Large quantities of grapefruit juice (over 1.2 l daily for 5 days) increased AUC of atorvastatin 2.5 fold and AUC of active (atorvastatin and metabolites).
** Ratio based on a single sample taken 8-16 h post dose
^ Total atorvastatin equivalent activity
Table 2. Effect of atorvastatin on the pharmacokinetics of co-administered medicinal products:
OD = once daily, SD = single dose, BID = twice daily
Increase is indicated as “↑”, decrease as "↓"
& Data given as % change represent % difference relative to atorvastatin alone (i.e., 0 % = no change)
* Co-administration of multiple doses of atorvastatin and phenazone showed little or no detectable effect in the clearance of phenazone.
4.6. Fertility, pregnancy and lactation
Women of childbearing potential
Women of child-bearing potential should use appropriate contraceptive measures during treatment with Lipercosyl (see section 4.3).
Pregnancy
Based on existing data with the individual components as described below, Lipercosyl is contraindicated during pregnancy (see section 4.3).
Atorvastatin
Safety in pregnant women has not been established. No controlled clinical trials with atorvastatin have been conducted in pregnant women. Rare reports of congenital anomalies following intrauterine exposure to HMG-CoA reductase inhibitors have been received. Animal studies have shown toxicity to reproduction (see section 5.3).
Maternal treatment with atorvastatin may reduce the foetal levels of mevalonate which is a precursor of cholesterol biosynthesis. Atherosclerosis is a chronic process, and ordinarily discontinuation of lipid-lowering medicinal products during pregnancy should have little impact on the long-term risk associated with primary hypercholesterolaemia.
For these reasons, atorvastatin should not be used in women who are pregnant, trying to become pregnant or suspected to be pregnant.
Perindopril
Epidemiological evidence regarding the risk of teratogenicity following exposure to ACE inhibitors during the first trimester of pregnancy has not been conclusive; however a small increase in risk cannot be excluded. Patients planning pregnancy should be changed to alternative anti-hypertensive treatments which have an established safety profile for use in pregnancy. When pregnancy is diagnosed, treatment with ACE inhibitors should be stopped immediately, and, if appropriate, alternative therapy should be started.
Exposure to ACE inhibitor therapy during the second and third trimesters is known to induce human foetoxicity (decreased renal function, oligohydramnios, skull ossification retardation) and neonatal toxicity (renal failure, hypotension, hyperkalaemia) (see section 5.3).
For these reasons the use of ACE inhibitors is not recommended during the first trimester of pregnancy. The use of ACE inhibitors is contraindicated during the 2nd and 3rd trimester of pregnancy.
Should exposure to ACE inhibitor have occurred from the second trimester of pregnancy, ultrasound check of renal function and skull is recommended. Infants whose mothers have taken ACE inhibitors should be closely observed for hypotension (see also sections 4.3 and 4.4).
Breast‑feeding
Based on existing data with the individual components as described below, Lipercosyl is contra-indicated during breast-feeding (see section 4.3).
Atorvastatin
It is not known whether atorvastatin or its metabolites are excreted in human milk. In rats, plasma concentrations of atorvastatin and its active metabolites are similar to those in milk (see section 5.3). Because of the potential for serious adverse reactions, women taking atorvastatin should not breast-feed their infants. Atorvastatin is contraindicated during breastfeeding (see section 4.3).
Perindopril
Because no information is available regarding the use of perindoprilduring breastfeeding, perindopril is not recommended and alternative treatments with better established safety profiles during breast-feeding are preferable, especially while nursing a newborn or preterm infant.
Fertility
There are no clinical data on fertility with the use of Lipercosyl.
Atorvastatin
In animal studies atorvastatin had no effect on male or female fertility (see section 5.3).
Perindopril
There was no effect on reproductive performance or fertility.
4.7. Effects on ability to drive and use machines
No studies have been performed on the effect of Lipercosyl on the ability to drive and use machines.
- Atorvastatin has negligible influence on the ability to drive and use machines.
- Perindopril has no direct influence on the ability to drive and use machines but individual reactions related to low blood pressure may occur in some patients, particularly at the start of treatment or in combination with another antihypertensive medication.
As a result the ability to drive or operate machinery may be impaired in patients taking Lipercosyl.
4.8. Undesirable effects
Summary of the profile
The most commonly reported adverse reactions with atorvastatin and perindopril given separately include: nasopharyngitis, hypersensitivity, hyperglycaemia, dizziness, headache, dysgeusia, paraesthesia, visual impairment, tinnitus, vertigo, hypotension, pharyngolaryngeal pain, epistaxis, cough, dyspnoea, nausea, vomiting, abdominal pain upper and lower, dyspepsia, diarrhoea, constipation, flatulence, rash, pruritus, joint swelling, pain in extremity, arthralgia, muscle spasms, myalgia, back pain, asthenia, liver function test abnormal, blood creatine kinase increased.
Tabulated list of adverse reactions
The following undesirable effects have been observed during treatment with atorvastatin and perindopril, or given separately and ranked under the MedDRA classification by body system and under heading of frequency using the following convention: Very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000); not known (cannot be estimated from the available data)).
| MedDRA System Organ Class | Undesirable effects | Frequency | |
|---|---|---|---|
| Atorvastatin | Perindopril | ||
| Infections and infestation | Nasopharyngitis | Common | - |
| Rhinitis | - | Very rare | |
| Blood and lymphatic system disorders | Thrombocytopenia | Rare | Very rare |
| Leukopenia/Neutropenia (see section 4.4) | - | Very rare | |
| Eosinophilia | - | Uncommon* | |
| Agranulocytosis /Pancytopenia (see section 4.4) | Very rare | ||
| Haemolytic anaemia in patients with a congenital deficiency of G-6PDH (see section 4.4) | - | Very rare | |
| Immune system disorders | Hypersensitivity | Commo | - |
| Anaphylaxis | Very rare | - | |
| Endocrine disorders | Syndrome of inappropriate antidiuretic hormone secretion (SIADH) | - | Rare |
| Metabolism and nutrition disorders | Hyperglycaemia | Common | - |
| Hypoglycaemia (see sections 4.4 and 4.5) | Uncommon | Uncommon* | |
| Hyponatraemia | - | Uncommon* | |
| Hyperkalaemia reversible on discontinuation (see section 4.4) | - | Uncommon* | |
| Anorexia | Uncommon | - | |
| Psychiatric disorders | Insomnia | Uncommon | - |
| Depression | - | Uncommon* | |
| Mood altered | - | Uncommon | |
| Sleep disorder | Uncommon | ||
| Nightmare | Uncommon | - | |
| Confusional state | - | Very rare | |
| Nervous system disorders | Somnolence | - | Uncommon* |
| Dizziness | Uncommon | Common | |
| Headache | Common | Common | |
| Dysgeusia | Uncommon | Common | |
| Syncope | - | Uncommon* | |
| Hypoaesthesia | Uncommon | - | |
| Paraesthesia | Uncommon | Common | |
| Peripheral neuropathy | Rare | - | |
| Stroke possible secondary to excessive hypotension in high-risk patients (see section 4.4) | - | Very rare | |
| Amnesia | Uncommon | - | |
| Eye disorders | Visual impairment | Rare | Common |
| Vision blurred | Uncommon | - | |
| Ear and labyrinth disorders | Tinnitus | Uncommon | Common |
| Vertigo | - | Common | |
| Hearing loss | Very Rare | - | |
| Cardiac disorders | Myocardial infarction, possibly secondary to excessive hypotension in high-risk patients (see section 4.4) | - | Very rare |
| Angina pectoris | - | Very rare | |
| Arrhythmia | - | Very rare | |
| Tachycardia | - | Uncommon* | |
| Palpitations | - | Uncommon* | |
| Vascular disorders | Hypotension (and effects related to hypotension) | - | Common |
| Vasculitis | - | Uncommon* | |
| Flushing | - | Rare* | |
| Raynaud’s phenomenon | - | Not known | |
| Respiratory, thoracic and mediastinal disorders | Pharyngolaryngeal pain | Common | - |
| Epistaxis | Common | - | |
| Cough | - | Common | |
| Dyspnoea | - | Common | |
| Bronchospasm | - | Uncommon | |
| Eosinophilic pneumonia | - | Very rare | |
| Gastro-intestinal disorders | Nausea | Common | Common |
| Vomiting | Uncommon | Common | |
| Abdominal pain upper and lower | Uncommon | Common | |
| Dyspepsia | Common | Common | |
| Diarrhoea | Common | Common | |
| Constipation | Common | Common | |
| Dry mouth | - | Uncommon | |
| Pancreatitis | Uncommon | Very rare | |
| Eructation | Uncommon | - | |
| Flatulence | Common | - | |
| Hepato-biliary disorders | Hepatitis either cytolytic or cholestatic (see section 4.4) | Uncommon | Very rare |
| Cholestasis | Rare | - | |
| Hepatic failure | Very rare | - | |
| Skin and subcutaneous tissue disorders | Rash | Uncommon | Common |
| Pruritus | Uncommon | Common | |
| Urticaria (see section 4.4) | Uncommon | Uncommon | |
| Hyperhidrosis | - | Uncommon | |
| Psoriasis aggravation | - | Rare* | |
| Alopecia | Uncommon | - | |
| Angioedema (see section 4.4) | Rare | Uncommon | |
| Pemphigoid | - | Uncommon* | |
| Stevens-Johnson syndrome | Rare | - | |
| Photosensitivity reaction | - | Uncommon* | |
| Toxic epidermal necrolysis | Rare | - | |
| Erythema multiforme | Rare | Very rare | |
* Frequency calculated from clinical trials for adverse events detected from spontaneous report.
As with other HMG-CoA reductase inhibitors elevated serum transaminases have been reported in patients receiving atorvastatin. These changes were usually mild, transient, and did not require interruption of treatment. Clinically important (>3 times upper normal limit) elevations in serum transaminases occurred in 0.8% patients on atorvastatin. These elevations were dose related and were reversible in all patients (see section 4.4).
Elevated serum creatine kinase (CK) levels greater than 3 times upper limit of normal occurred in 2.5% of patients on atorvastatin, similar to other HMG-CoA reductase inhibitors in clinical trials. Levels above 10 times the normal upper range occurred in 0.4% atorvastatin -treated patients (see section 4.4).
The following adverse events have been reported with some statins:
- Sexual dysfunction.
- Depression.
- Exceptional cases of interstitial lung disease, especially with long term therapy (see section 4.4).
- Diabetes Mellitus: Frequency will depend on the presence or absence of risk factors (fasting blood glucose ≥5.6 mmol/L, BMI >30 kg/m², raised triglycerides, history of hypertension).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via: HPRA Pharmacovigilance, Website: www.hpra.ie
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.