LIVTENCITY Film-coated tablet Ref.[50474] Active ingredients: Maribavir
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Takeda Pharmaceuticals International AG Ireland Branch, Block 3 Miesian Plaza, 50-58 Baggot Street Lower, Dublin 2, D02 Y754, Ireland E-mail: medinfoEMEA@takeda.com
4.3. Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
Co-administration with ganciclovir or valganciclovir (see section 4.5).
4.4. Special warnings and precautions for use
Virologic failure during treatment and relapse post-treatment
Virologic failure can occur during and after treatment with LIVTENCITY. Virologic relapse during the post-treatment period usually occurred within 4-8 weeks after treatment discontinuation. Some maribavir pUL97 resistance-associated substitutions confer cross-resistance to ganciclovir and valganciclovir. CMV DNA levels should be monitored and resistance mutations should be investigated in patients who do not respond to treatment. Treatment should be discontinued if maribavir resistance mutations are detected.
CMV disease with CNS involvement
LIVTENCITY was not studied in patients with CMV CNS infection. Based on nonclinical data, CNS penetration of maribavir is expected to be low compared to plasma levels (section 5.2 and 5.3). Therefore, LIVTENCITY is not expected to be effective in treating CMV CNS infections (e.g. meningo-encephalitis).
Use with immunosuppressants
LIVTENCITY has the potential to increase the concentrations of immunosuppressants that are cytochrome P450 (CYP)3A/P-gp substrates with narrow therapeutic margins (including tacrolimus, cyclosporine, sirolimus and everolimus). The plasma levels of these immunosuppressants must be frequently monitored throughout treatment with LIVTENCITY, especially following initiation and after discontinuation of LIVTENCITY, and doses should be adjusted, as needed (see sections 4.5, 4.8 and 5.2).
Risk of adverse reactions or reduced therapeutic effect due to medicinal product interactions
The concomitant use of LIVTENCITY and certain medicinal products may result in known or potentially significant medicinal product interactions, some of which may lead to:
- possible clinically significant adverse reactions from greater exposure of concomitant medicinal products.
- reduced therapeutic effect of LIVTENCITY.
See Table 1 for steps to prevent or manage these known or potentially significant medicinal product interactions, including dosing recommendations (see sections 4.3 and 4.5).
Sodium content
This medicinal product contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’.
4.5. Interaction with other medicinal products and other forms of interaction
Effect of other medicinal products on maribavir
Maribavir is primarily metabolised by CYP3A, and medicinal products that induce or inhibit CYP3A are expected to affect the clearance of maribavir (see section 5.2).
Co-administration of maribavir and medicinal products that are inhibitors of CYP3A may result in increased plasma concentrations of maribavir (see section 5.2). However, no dose adjustment is needed when maribavir is co-administered with CYP3A inhibitors.
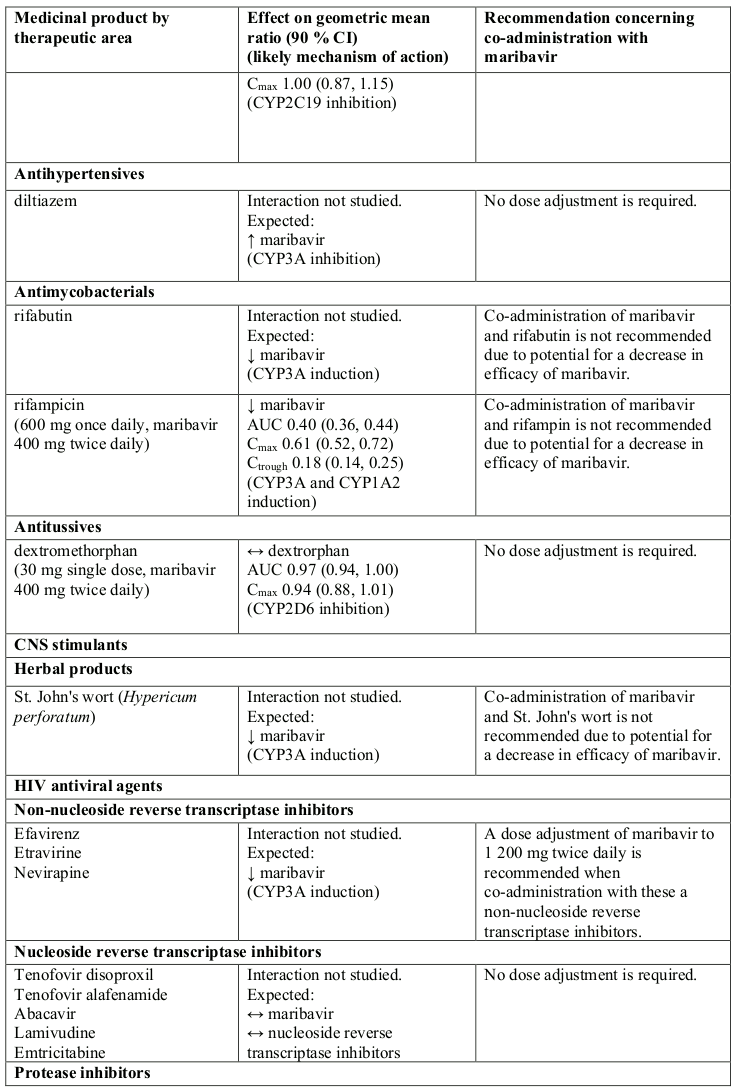
Concomitant administration of strong or moderate CYP3A inducers, (such as rifampicin, rifabutin, carbamazepine, phenobarbital, phenytoin, efavirenz and St John’s wort), is expected to significantly decrease maribavir plasma concentrations, which may result in decrease in efficacy. Therefore, alternative medicinal products with no CYP3A induction potential should be considered. Coadministration of maribavir with strong cytochrome P450 3A (CYP3A) inducers rifampicin, rifabutin or St. John’s wort is not recommended.
If co-administration of maribavir with other strong or moderate CYP3A inducers (e.g., carbamazepine, efavirenz, phenobarbital and phenytoin) cannot be avoided, the maribavir dose should be increased to 1 200 mg twice daily (see sections 4.2 and 5.2).
Effect of maribavir on other medicinal products
Co-administration of maribavir with valganciclovir and ganciclovir is contraindicated (see section 4.3). LIVTENCITY may antagonise the antiviral effect of ganciclovir and valganciclovir by inhibiting human CMV UL97 serine/threonine kinase, which is required for activation/phosphorylation of ganciclovir and valganciclovir (see sections 4.3 and 5.1).
At therapeutic concentrations, clinically relevant interactions are not expected when maribavir is coadministered with substrates of CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2E1, 2D6, and 3A4; UGT1A1, 1A4, 1A6, 1A9, 2B7; bile salt export pump (BSEP); multidrug and toxin extrusion protein (MATE)/2K; organic anion transporters (OAT)1; organic cation transporters (OCT)1 and OCT2; organic anion transporting polypeptide (OATP)1B1 and OATP1B3 based on in vitro and clinical interaction results (Table 1 and section 5.2).
Maribavir acted as an inducer of CYP1A2 enzyme in vitro. There are no clinical data available to exclude an interaction risk via CYP1A2 induction in vivo. Therefore, the concomitant administration of maribavir and medicinal products that are sensitive substrates of CYP1A2 with a narrow therapeutic window (e.g., tizanidine and theophylline) should be avoided due to the risk for lack of efficacy of CYP1A2 substrates.
Co-administration of maribavir increased plasma concentrations of tacrolimus (see Table 1). When the immunosuppressants tacrolimus, cyclosporine, everolimus or sirolimus are co-administered with maribavir, immunosuppressant levels should be frequently monitored throughout treatment with maribavir, especially following initiation and after discontinuation of maribavir and dose adjusted, when needed (see sections 4.4 and Table 1).
Maribavir inhibited P-gp transporter in vitro at clinically relevant concentrations. In a clinical study, co-administration of maribavir increased plasma concentrations of digoxin (see Table 1). Therefore, caution should be exercised when maribavir and sensitive P-gp substrates (e.g., digoxin, dabigatran) are co-administered. Serum digoxin concentrations should be monitored, and dose of digoxin may need to be reduced, as needed (see Table 1).
Maribavir inhibited BCRP transporter in vitro at clinically relevant concentrations. Therefore, co-administration of maribavir with sensitive BCRP substrates such as rosuvastatin, is expected to increase their exposure and lead to undesirable effects.
In vitro, maribavir inhibits OAT3, therefore, plasma concentrations of medicinal products transported by OAT3 may be increased (e.g.: ciprofloxacin, imipenem, and cilastin).
In vitro, maribavir inhibits MATE1. There are no clinical data available whether the co-administration of maribavir with sensitive MATE1 substrates (e.g., metformin) could potentially lead to clinically relevant interactions.
General information
If dose adjustments of concomitant medicinal products are made due to treatment with maribavir, doses should be readjusted after treatment with maribavir is completed. Table 1 provides a listing of established or potentially clinically significant medicinal product interactions. The medicinal product interactions described are based on studies conducted with maribavir or are predicted medicinal product interactions that may occur with maribavir (see sections 4.4 and 5.2).
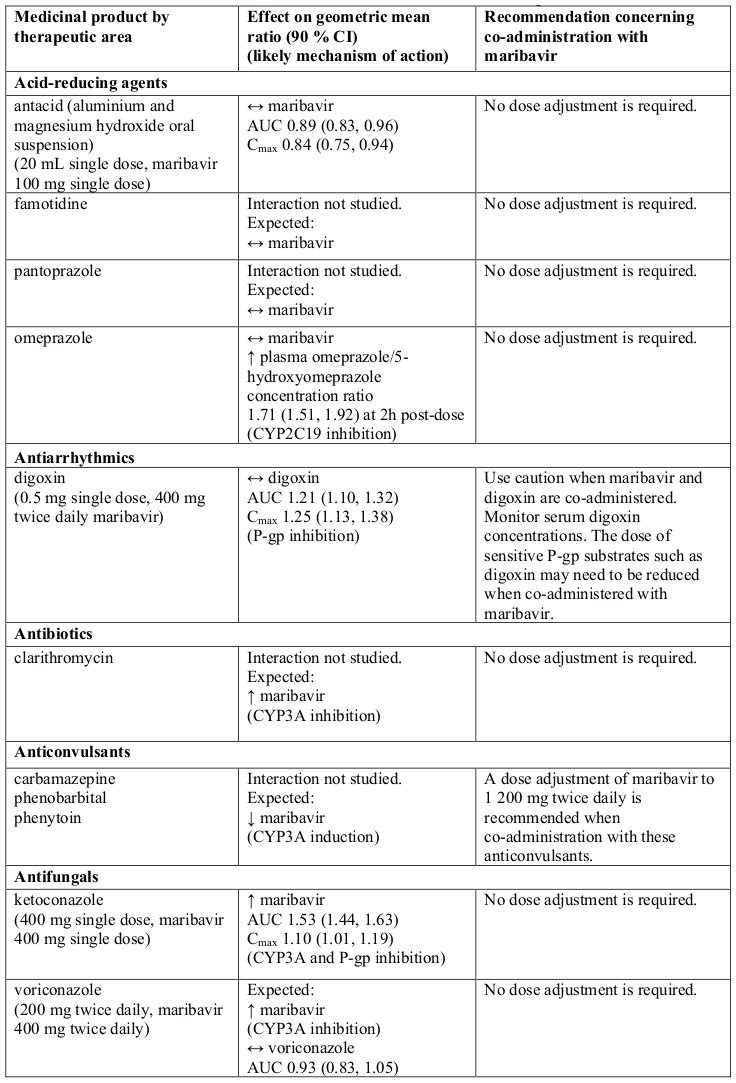
Table 1. Interactions and dose recommendations with other medicinal products:
↑ = increase, ↓ = decrease, ↔ = no change
CI = Confidence Interval; SD = Single Dose; QD = Once Daily; BID = Twice Daily
* AUC0-∞ for single dose, AUC0-12 for twice daily dose daily.
Note: the table is not extensive but provides examples of clinically relevant interactions.
a Refer to the respective prescribing information.
Paediatric population
Interaction studies have only been performed in adults.
4.6. Fertility, pregnancy and lactation
Pregnancy
There are no data of maribavir use in pregnant women. Studies in animals have shown reproductive toxicity (see section 5.3). LIVTENCITY is not recommended during pregnancy and in women of childbearing potential not using contraception.
Maribavir is not expected to affect the plasma concentrations of systemically acting oral contraceptive steroids (see Section 4.5).
Breast-feeding
It is unknown whether maribavir or its metabolites are excreted in human milk. A risk to the suckling child cannot be excluded. Breast-feeding should be discontinued during treatment with LIVTENCITY.
Fertility
Fertility studies were not conducted in humans with LIVTENCITY. No effects on fertility or reproductive performance were noted in rats in a combined fertility and embryofoetal development study, however, a decrease in sperm straight line velocity was observed at doses ≥100 mg/kg/day (which is estimated to be <1 times the human exposure at the recommended human dose [RHD]). There were no effects on reproductive organs in either males or females in nonclinical studies in rats and monkeys (see section 5.3).
4.7. Effects on ability to drive and use machines
LIVTENCITY has no influence on the ability to drive and use machines.
4.8. Undesirable effects
Summary of the safety profile
Adverse events were collected during the treatment phase and follow-up phase through Study Week 20 in the Phase 3 study (see section 5.1). The mean exposures (SD) for LIVTENCITY was 48.6 (13.82) days with a maximum of 60 days. The most commonly reported adverse reactions occurring in at least 10% of subjects in the LIVTENCITY group were: taste disturbance (46%), nausea (21%), diarrhoea (19%), vomiting (14%) and fatigue (12%). The most commonly reported serious adverse reactions were diarrhoea (2%) and nausea, weight decreased, fatigue, immunosuppressant drug concentration level increased, and vomiting (all occurring at >1%).
Tabulated list of adverse reactions
The adverse reactions are listed below by body system organ class and frequency. Frequencies are defined as follows: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1 000 to <1/100), rare (≥1/10 000 to <1/1 000) or very rare (<1/10 000).
Table 2. Adverse reactions identified with LIVTENCITY:
| System Organ Class | Frequency | Adverse reactions |
|---|---|---|
| Nervous system disorders | Very common | Taste disturbance* |
| Common | Headache | |
| Gastrointestinal disorders | Very Common | Diarrhoea, Nausea, Vomiting |
| Common | Abdominal pain upper | |
| General disorders and administration site conditions | Very common | Fatigue |
| Common | Decreased appetite | |
| Investigations | Common | Immunosuppressant drug level increased*, Weight decreased |
Description of selected adverse reactions*
Taste disturbance
Taste disturbance (comprised of the reported preferred terms ageusia, dysgeusia, hypogeusia and taste disorder) occurred in 46% of patients treated with LIVTENCITY. These events rarely led to discontinuation of LIVTENCITY (0.9%) and, for most patients, resolved while patients remained on therapy (37%) or within a median of 7 days (Kaplan-Meier estimate, 95% CI: 4-8 days) after treatment discontinuation.
Increases in plasma levels of immunosuppressants
Immunosuppressant drug level increase (comprised of the preferred terms immunosuppressant drug level increased and drug level increased) occurred in 9% of patients treated with LIVTENCITY. LIVTENCITY has the potential to increase the drug concentrations of immunosuppressants that are CYP3A and/or P-gp substrates with narrow therapeutic ranges (including tacrolimus, cyclosporine, sirolimus and everolimus). (See sections 4.4, 4.5 and 5.2).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.