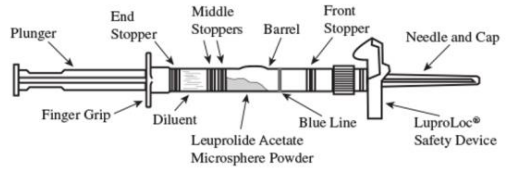
LUPRON DEPOT Powder for solution for injection Ref.[109539] Active ingredients: Leuprorelin
Source: FDA, National Drug Code (US) Revision Year: 2023
1. Indications and Usage
1.1 Endometriosis
Monotherapy
LUPRON DEPOT 3.75 mg is indicated for management of endometriosis, including pain relief and reduction of endometriotic lesions.
In Combination with Norethindrone Acetate
LUPRON DEPOT 3.75 mg in combination with norethindrone acetate is indicated for initial management of the painful symptoms of endometriosis and for management of recurrence of symptoms.
Use of norethindrone acetate in combination with LUPRON DEPOT 3.75 mg is referred to as add-back therapy, and is intended to reduce the loss of bone mineral density (BMD) and reduce vasomotor symptoms associated with use of LUPRON DEPOT 3.75 mg.
Limitations of Use
The total duration of therapy with LUPRON DEPOT 3.75 mg plus add-back therapy should not exceed 12 months due to concerns about adverse impact on bone mineral density [see Dosage and Administration (2.1) and Warnings and Precautions (5.1)].
1.2 Uterine Leiomyomata (Fibroids)
LUPRON DEPOT 3.75 mg, used concomitantly with iron therapy, is indicated for the preoperative hematologic improvement of women with anemia caused by fibroids for whom three months of hormonal suppression is deemed necessary.
Consider a one-month trial period on iron alone, as some women will respond to iron alone [see Clinical Studies (14.2)]. LUPRON DEPOT 3.75 mg may be added if the response to iron alone is considered inadequate.
Limitations of Use
LUPRON DEPOT 3.75 mg is not indicated for combination use with norethindrone acetate add-back therapy for the preoperative hematologic improvement of women with anemia caused by heavy menstrual bleeding due to fibroids [see Dosage and Administration (2.1)].
2. Dosage and Administration
2.1 Important Use Information
LUPRON DEPOT 3.75 mg must be administered by a healthcare professional.
LUPRON DEPOT 3.75 mg for 1-month administration has different release characteristics than LUPRON 11.25 mg for 3-month administration and is dosed differently.
- Do not substitute LUPRON DEPOT 3.75 mg for LUPRON DEPOT 11.25 mg.
- Do not administer LUPRON DEPOT 3.75 mg more frequently than once a month.
- Do not give a fractional dose of the LUPRON DEPOT 11.25 mg 3-month formulation as it is not equivalent to a single dose of the LUPRON DEPOT 3.75 mg.
- Do not give a triple dose of the LUPRON DEPOT 3.75 mg, as it is not equivalent to a single dose of the LUPRON DEPOT 11.25 mg 3-month formulation.
Endometriosis
The initial and retreatment dosage regimens for LUPRON DEPOT 3.75 mg for the management of women with endometriosis are outlined in Table 1.
Table 1. LUPRON DEPOT 3.75 mg, Management of Endometriosis:
| Treatment Phase | LUPRON DEPOT 3.75 mg Dosing | Maximum Treatment Duration |
| Initial Treatment 1 | 3.75 mg IM every 1 month for 1 to 6 doses | 6 months |
| Retreatment 2 | 3.75 mg IM every 1 month for 1 to 6 doses | 6 months |
| 12 MONTHS 3 TOTAL TREATMENT DURATION |
1May use LUPRON DEPOT 3.75 mg with or without norethindrone acetate 5 mg tablet taken daily.
2Use LUPRON DEPOT 3.75 mg with norethindrone acetate for retreatment 5 mg tablet taken daily [see Warnings and Precautions (5.1)] and assess bone mineral density (BMD) prior to retreatment.
3Treatment should not exceed 12 months due to concerns about adverse impact on bone mineral density.
Fibroids
The recommended dosage of LUPRON DEPOT 3.75 mg is one IM injection every month for up to three months.
2.2 Reconstitution and Administration for Injection of LUPRON DEPOT
- Reconstitute and administer the lyophilized microsphere as a single IM injection as directed below. Visually inspect the drug product for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Inject the LUPRON DEPOT 3.75 mg suspension immediately or discard if not used within two hours as the suspension does not contain a preservative.
1. Visually inspect the LUPRON DEPOT 3.75 mg powder. Do not use the syringe if clumping or caking is evident. A thin layer of powder on the wall of the syringe is considered normal prior to mixing with the diluent. The diluent should appear clear.
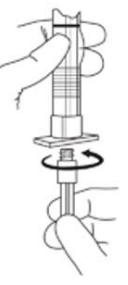
2. To prepare for injection, screw the white plunger into the end stopper until the stopper begins to turn (see Figure A and Figure B).
Figure A:
Figure B:
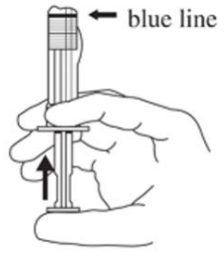
3. Hold the syringe UPRIGHT. Release the diluent by SLOWLY PUSHING the plunger for 6 to 8 seconds until the first middle stopper is at the blue line in the middle of the barrel (see Figure C).
Figure C:
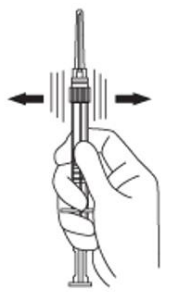
4. Keep the syringe upright. Mix the microsphere powder thoroughly by gently shaking the syringe until the powder forms a uniform suspension. The suspension will appear milky. If the powder adheres to the stopper or caking/clumping is present, tap the syringe with your finger to disperse. Do not use if any of the powder has not gone into suspension (see Figure D).
Figure D:
5. Keep the syringe upright. With the opposite hand pull the needle cap upward without twisting.
6. Keep the syringe upright. Advance the plunger to expel the air from the syringe. The syringe is now ready for injection.
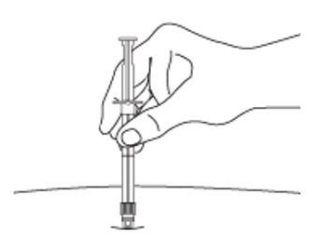
7. After cleaning the injection site with an alcohol swab, administer the IM injection by inserting the needle at a 90-degree angle into the gluteal area, anterior thigh, or deltoid. Injection sites should be alternated (see Figure E).
Figure E:
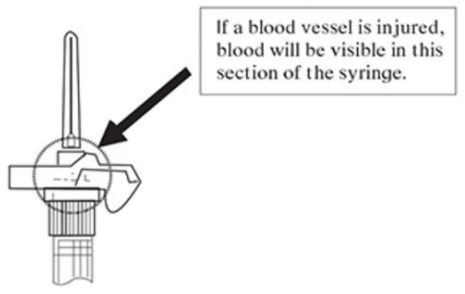
Note: If a blood vessel is accidentally penetrated, aspirated blood will be visible just below the luer lock (see Figure F) and can be seen through the transparent LuproLoc safety device. If blood is present, remove the needle immediately. Do not inject the medication.
Figure F:
8. Inject the entire contents of the syringe intramuscularly.
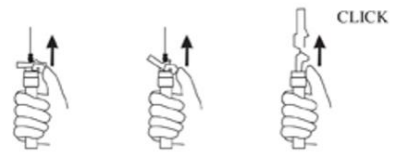
9. Withdraw the needle. Once the syringe has been withdrawn, immediately activate the LuproLoc safety device by pushing the arrow on the lock upward towards the needle tip with the thumb or finger, as illustrated, until the needle cover of the safety device over the needle is fully extended and a click is heard or felt (see Figure G).
Figure G:
10. Dispose of the syringe according to local regulations/procedures.
16.2. Storage and Handling
Store between 20° to 25°C (68° to 77°F). Excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.