LYTENAVA Solution for injection Ref.[110288] Active ingredients: Bevacizumab
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: Outlook Therapeutics Limited, 10 Earlsfort Terrace, Dublin 2, D02 T380, Ireland
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Ophthalmolgicals, antineovascularisation agents
ATC code: S01LA08
Mechanism of action
Bevacizumab gamma is a recombinant humanised IgG1 monoclonal antibody (mAb) for human vascular endothelial growth factor (VEGF).
Bevacizumab gamma binds VEGF and prevents the interaction of VEGF to its receptors (Flt-1 and KDR) on the surface of endothelial cells. Bevacizumab gamma is a human VEGF inhibitor that binds to all isoforms of VEGF-A, thereby preventing interaction with receptors VEGFR-1 and VEGFR-2. By inhibiting VEGF-A, bevacizumab gamma suppresses endothelial cells proliferation, neovascularization, and vascular permeability. Inhibition of angiogenesis works to block the growth of abnormal blood vessels in the back of the eye.
Pharmacodynamic effects
Neovascular AMD
In the NORSE TWO study, anatomical parameters related to leakage of blood and fluid that characterise choroidal neovascularisation (CNV) were part of the disease activity assessments. A mean decrease in central retinal thickness (CRT) of 119.7 microns at month 11 compared to baseline was observed in patients receiving monthly 1.25 mg bevacizumab gamma intravitreal injections.
Immunogenicity
No evidence of anti-drug antibodies (ADA) impact on pharmacokinetics, efficacy or safety was observed, however, data are still limited.
Clinical efficacy and safety
The efficacy and safety of bevacizumab gamma was assessed in two randomised, multicentre, doublemasked, active controlled Phase III studies (NORSE ONE and NORSE TWO) in patients with nAMD. In NORSE ONE, both patients with previously treated and treatment naive study eyes were enrolled and a total of 61 patients were randomized 1:1 (31 subjects in the bevacizumab group and 30 subjects in the ranibizumab group). Patient ages ranged from 61 to 97 years, with a mean age of 79 years; 97% of patients were over 65 years. In NORSE TWO, treatment naive study eyes were enrolled and a total of 228 patients were randomised 1:1 (113 subjects in the bevacizumab gamma group and 115 subjects in the ranibizumab group). Patient ages ranged from 54 to 98 years, with a mean age of 79 years; 95% of patients were over 65 years.
In both studies, patients randomised to receive bevacizumab gamma were administered at a dose of 1.25 mg by intravitreal injection in the study eye every month for 12 months. Patients randomised to ranibizumab control were administered at a dose of 0.5 mg by intravitreal injection in the study eye every month for 3 months (i.e. on Days 0, 30, and 60) followed by every 90 days (i.e. on Days 150 and 240), which was a sublabel dosing regimen. In total, 5 injections in the ranibizumab arm were compared to 11 injections in the bevacizumab gamma arm for the assessment of the primary endpoint. The primary endpoint was assessed at the Month 11 visit, which was approximately 30 days after the last bevacizumab gamma dose and 90 days after the last ranibizumab dose.
The primary endpoint was assessed at the Month 11 visit, which was approximately 30 days after the last bevacizumab gamma dose and 90 days after the last ranibizumab dose. The primary endpoint in both studies was the proportion of subjects who gained ≥15 letters in best corrected visual acuity (BCVA) from baseline to month 11, as measured by the early treatment diabetic retinopathy study (ETDRS) letter score, with the primary objective being to demonstrate the efficacy of bevacizumab gamma in a nAMD population. Secondary endpoints evaluated the change from baseline at month 11 in mean BCVA and the proportion of subjects who lost fewer than 15 letters in BCVA.
Results
The proportion of subjects in the NORSE ONE study who achieved an increase of ≥15 letters in BCVA from baseline to 11 months was 7.7% vs 20.8%, respectively, in the bevacizumab gamma and ranibizumab groups, (risk difference of 13.14% [95% CI = −35.50%, 7.65%]).Based on the primary endpoint the NORSE ONE study failed to demonstrate superiority of bevacizumab gamma over ranibizumab.
The NORSE TWO study met its primary efficacy endpoint and bevacizumab gamma demonstrated efficacy. The proportion of subjects who achieved an increase of ≥15 letters in BCVA from baseline to 11 months was 41.7% and 23.1% respectively, in the bevacizumab gamma and ranibizumab groups (risk difference of 18.59% [95% CI = 4.42%, 30.86%]). The primary efficacy analysis was statistically significant, in favour of bevacizumab gamma.
The efficacy of bevacizumab gamma was further supported when evaluating the change from baseline to month 11 in mean BCVA. The difference between the treatments and its corresponding 95% CI was 3.805 (-0.016, 7.626) BCVA letters.
Table 2. NORSE TWO primary and secondary efficacy endpoints – responder analysis:
| Ranibizumab (N=115) | Bevacizumab gamma (N=113) | |
|---|---|---|
| Primary Endpoint | ||
| Subjects gaining ≥15 letters from baseline at 11 months, n/N (%) | 24/104 (23.1) | 45/108 (41.7) |
| Risk difference | 18.59% | |
| 95% CI | 4.42%; 30.86% | |
| Secondary Endpoints | ||
| BCVA mean change from baseline to 11 months, mean (SD) | 5.8 (14.80) | 11.2 (12.19) |
| LS mean change difference | 3.805 | |
| 95% CI | -0.016, 7.626 | |
| Subjects gaining ≥10 letters from baseline at 11 months, n/N (%) | 36/104 (34.6) | 61/108 (56.5) |
| Risk difference | 21.87% | |
| 95% CI | 7.26%, 34.87% | |
| Subjects gaining ≥5 letters from baseline at 11 months, n/N (%) | 53/104 (51.0) | 74/108 (68.5) |
| Risk difference | 17.56% | |
| 95% CI | 3.15%, 30.52% | |
| Subjects losing <15 letters from baseline at 11 months, n/N (%) | 86/104 (82.7) | 101/108 (93.5) |
| Risk difference | 10.83% | |
| 95% CI | 1.68%, 20.44% | |
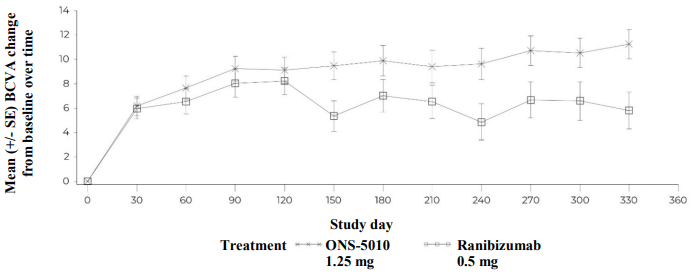
Figure 1. NORSE TWO - Best-corrected visual acuity change from baseline over time*:
* ONS-5010 (bevacizumab gamma) was dosed monthly for 12 months; ranibizumab was dosed every month for 3 months (i.e. on Days 0, 30, and 60) followed by every 90 days (i.e. on Days 150 and 240). In total, 5 injections in the ranibizumab arm were compared to 11 injections in the ONS-5010 arm for the assessment of the efficacy endpoints.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with bevacizumab gamma in all subsets of the paediatric population in neovascular AMD (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Bevacizumab gamma is administered intravitreally to exert local effects in the eye.
Following a single dose of 2 mg/kg intravenous infusion of bevacizumab gamma in 45 healthy male volunteers, the peak concentration was reached at 2 hours. The geometric mean Cmax and total exposure (AUC0-t) values were 40 µg/mL and 12 148 h•µg/mL, respectively.
In general, the serum PK following intravitreal administration of bevacizumab gamma, was significantly lower than that seen following intravenous administration. No PK parameters could be characterised from the generated clinical data.
5.3. Preclinical safety data
In a review of the preclinical safety evaluation of bevacizumab, female cynomolgus monkeys administered intravenous bevacizumab twice weekly for 13 weeks had decreased ovarian weight and a microscopic correlate of absence of corpora lutea at ≥10 mg/kg that was reversible after a 4-week recovery period. Ovarian effects can be attributed to a direct result of the local inhibition of VEGF on active angiogenesis, which is profound in the ovary.
No carcinogenicity or mutagenicity data are available.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.