MIEBO Ophthalmic solution Ref.[107374] Active ingredients: Perfluorohexyloctane
Source: FDA, National Drug Code (US) Revision Year: 2023
12.1. Mechanism of Action
Perfluorohexyloctane, a semifluorinated alkane, contains 6 perfluorinated carbon atoms and 8 hydrogenated carbon atoms. Perfluorohexyloctane forms a monolayer at the air-liquid interface of the tear film which can be expected to reduce evaporation. The exact mechanism of action for MIEBO in DED is not known.
12.3. Pharmacokinetics
The pharmacokinetics of perfluorohexyloctane following topical ocular administration of MIEBO has not been quantitatively characterized in humans. A single pharmacokinetic (PK) study was conducted that showed low systemic perfluorohexyloctane blood levels after topical ocular administration. Perfluorohexyloctane was not metabolized by human liver microsomes in vitro.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been conducted to evaluate the carcinogenic potential of perfluorohexyloctane.
Perfluorohexyloctane was not mutagenic or clastogenic in a standard battery of genotoxicity tests, including a bacterial mutagenicity assay (Ames assay), an in vitro chromosome aberration assay using human peripheral lymphocytes, and an in vivo bone marrow micronucleus assay in rats.
14. Clinical Studies
In two randomized, multicenter, double-masked, saline-controlled trials (GOBI and MOJAVE), a total of 1,217 patients with a history of DED and clinical signs of meibomian gland dysfunction were randomized to MIEBO or saline 0.6% (1:1 ratio) to evaluate safety and efficacy after receiving MIEBO four times daily (QID) for 57 days.The mean age of the 614 patients who received MIEBO was 57 years (range, 19-87 years). The majority of patients were female (76%).
Effects on Signs of Dry Eye Disease
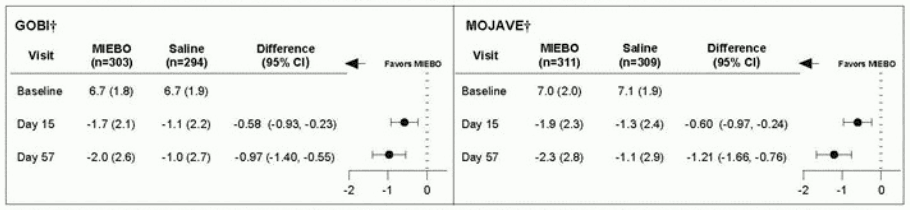
Total corneal fluorescein staining (tCFS) was recorded at each study visit using a standardized grading system of 0-3 for each of the five areas on the cornea (inferior, superior, central, nasal, and temporal), totaling a maximum tCFS score for each eye of 15. The average baseline tCFS was approximately 6.7 in GOBI and 7.0 in MOJAVE. At Days 15 and 57, a statistically significant reduction in tCFS favoring MIEBO was observed in both studies (Figure 1).
Figure 1. Mean Change (Standard Deviation) from Baseline and Treatment Difference (MIEBO-Saline) in Total Corneal Fluorescein Staining (Study Eye) in 8-Week Study in Patients with Dry Eye Disease:
† A Phase 3, Multi-Center, Randomized, Double-Masked, Saline-Controlled Trial to Evaluate the Effect of NOV03 (Perfluorohexyloctane) on Signs and Symptoms of Dry Eye Disease Associated with Meibomian Gland Dysfunction
Effects on Symptoms of Dry Eye Disease
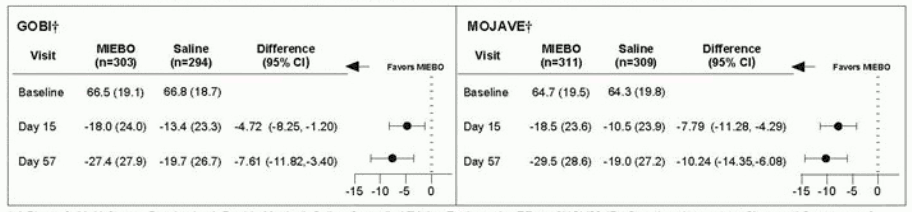
Eye dryness score was rated by patients using a visual analogue scale (VAS) (0=no discomfort, 100=maximal discomfort) at each study visit. The baseline VAS eye dryness average score was approximately 67 in GOBI and 65 in MOJAVE. At Days 15 and 57, a statistically significant reduction in VAS eye dryness score favoring MIEBO was observed in both studies (Figure 2).
Figure 2. Mean Change (Standard Deviation) from Baseline and Treatment Difference (MIEBO-Saline) in Eye Dryness (Study Eye) in 8-week Study in Patients with Dry Eye Disease:
† A Phase 3, Multi-Center, Randomized, Double-Masked, Saline-Controlled Trial to Evaluate the Effect of NOV03 (Perfluorohexyloctane) on Signs and Symptoms of Dry Eye Disease Associated with Meibomian Gland Dysfunction
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.