MVABEA Suspension for injection Ref.[50431] Active ingredients: Ebola vaccine
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Janssen-Cilag International NV, Turnhoutseweg 30, B-2340 Beerse, Belgium
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Vaccine, other viral vaccines
ATC code: J07BX02
Mechanism of action
Mvabea is a recombinant, non-replicating in human cells, Modified Vaccinia Ankara – Bavarian Nordic (MVA-BN) vectored multivalent Filovirus vaccine that encodes the Zaire ebolavirus Mayinga variant GP, Sudan ebolavirus Gulu variant GP, Taï Forest ebolavirus nucleoprotein, Marburg marburgvirus Musoke variant GP. The EBOV GP encoded by Zabdeno has 100% homology to the one encoded by Mvabea. Following administration, the EBOV GP is expressed locally and stimulates an immune response.
Efficacy
In the absence of efficacy data from clinical studies, the efficacy of the 2-dose primary vaccination regimen has been assessed through challenge studies in non-human primates (NHP, Cynomolgus macaques, Macaca fascicularis), the most relevant animal model for EBOV disease. The 2-dose primary vaccination regimen administered at an interval of 8 weeks was protective down to a first dose of 2 × 109 virus particle (VP) of Zabdeno, in combination with 1 × 108 Inf.U of Mvabea, in a lethal intramuscular EBOV Kikwit NHP challenge model. Humoral immune responses, as measured by the level of EBOV GP-binding antibodies, were strongly correlated to survival in NHP. Protective effect in humans has been inferred through comparison of EBOV GP-binding antibody concentrations (immunobridging).
Clinical immunogenicity
In the absence of efficacy data from clinical studies, the protective effect of the vaccine has been inferred from immunogenicity data. Data from 5 clinical studies conducted in Europe, the United States, and Africa in 764 adults 18 to 50 years of age who had received the 2-dose primary vaccination regimen at the 8-week interval were used in this analysis. Anti-EBOV GP binding antibodies were correlated with a protective effect against a rapidly progressing fully lethal Ebola virus infection in non-human primates. The human immune responses measured 21 days post-dose 2 were associated with an increase of the predicted survival probability from 0% (i.e., fully lethal) to 53.4% (98.68% CI: 33.8%; 70.9%) using the animal model. Based on this analysis, the Zabdeno, Mvabea vaccine regimen can be anticipated to have a protective effect against EBOV disease in humans. Although the relationship between antibody titre and survival has been studied only in adult NHP, immunobridging performed on paediatric subjects, the elderly and HIV-infected subjects suggests that the potential protective effects for these populations are consistent with the one estimated in adults.
Immunogenicity
Immunogenicity data are presented for a total of 842 adults and 509 children (1 to 17 years of age) who had received the 2-dose primary vaccination regimen in Phase II and III clinical studies: study EBL2001 in the UK and France, studies EBL3002 and EBL3003 in the United States, study EBL2002 in Uganda, Kenya, Burkina Faso and Cote d’Ivoire, and study EBL3001 in Sierra Leone. The concentrations of EBOV GP-specific binding antibodies were measured approximately 3 weeks after completion of the 2-dose primary vaccination regimen. These are presented as geometric mean concentrations (GMC).
Immunogenicity data in adults after the 2-dose primary vaccination regimen
The immune response to the 2-dose primary vaccination regimen given in an 8-week interval was assessed in 5 Phase II and III studies conducted in Europe, Africa and the USA (see Table 3). In all studies, 98% to 100% of study participants mounted a binding antibody response to EBOV GP, defined as more than 2.5-fold increase in binding antibody concentration over baseline value.
Table 3. EBOV GP-specific Binding Antibody Responses to the Zabdeno, Mvabea 2-dose Vaccine Regimen in Adults (8 week interval): GMC EU/mL (95% CI):
| Study | Baseline | 21 days post-dose 2 | 6 months post-dose 2 | 10 months post-dose 2 |
|---|---|---|---|---|
| EBL2001 | (N=70) <LLOQ (<LLOQ; <LLOQ) | (N=69) 10131 (8554; 11999) | - | (N=50) 1205 (971; 1497) |
| EBL2002 | (N=134) 39 (<LLOQ; 48) | (N=136) 7518 (6468; 8740) | - | (N=133) 342 (291; 401) |
| EBL3001 | (N=231) 68 (56; 81) | (N=224) 3976 (3517; 4495) | - | (N=199) 268 (234; 307) |
| EBL3002 | (N=140) <LLOQ (<LLOQ; <LLOQ) | (N=135) 11054 (9673; 12633) | (N=131) 1263 (1100; 1450) | - |
| EBL3003 | (N=258) <LLOQ (<LLOQ; <LLOQ) | (N=254) 11052 (9959; 12265) | (N=244) 1151 (1024; 1294) | - |
Data shown for vaccinated participants who received the 2-dose vaccine regimen in the Per Protocol Analysis Set.
EU = ELISA Units
CI = Confidence interval
N = Number of participants with data
LLOQ = Lower limit of quantification
The interval between doses in these studies was 8 weeks +/- 3 days. While the immunogenicity of vaccine regimens with a longer interval between doses up to 69 weeks (483 days) was similar, vaccine regimens with an interval of 4 weeks were less immunogenic.
Following the 2-dose primary vaccination regimen with an 8-week interval, GMCs EU/mL (95% CI) of 5283 (4094; 6817) were observed in HIV-infected adults on antiretroviral therapy, with CD4+ cells >350 cells/microlitre and no signs of immunosuppression (N=59).
Immunogenicity data in children after the 2-dose primary vaccination regimen
The immune response to the 2-dose primary vaccination regimen given in an 8-week interval was assessed in children (1 to 17 years of age) in two studies conducted in Africa (see Table 4). In the two studies, 98% to 100% of study participants mounted a binding antibody response to EBOV GP. Immune responses in children were higher than those observed in adults in the same studies.
Table 4. EBOV GP-specific Binding Antibody Responses to the Zabdeno, Mvabea 2-dose Vaccine Regimen in Children 1 to 17 years of age (8 week interval): GMC EU/mL (95% CI):
| Age | Study | Baseline | 21 days post-dose 2 | 6 months post-dose 2 | 10 months post-dose 2 |
|---|---|---|---|---|---|
| 1-3 years | EBL3001 | (N=123) <LLOQ (<LLOQ; <LLOQ) | (N=124) 22568 (18426; 27642) | (N=122) 713 (598; 849) | (N=120) 750 (629; 894) |
| 4-11 years | EBL2002 | (N=52) <LLOQ (<LLOQ; <LLOQ) | (N=53) 17388 (12973; 23306) | (N=53) 715 (602; 851) | (N=54) 637 (529; 767) |
| EBL3001 | (N=130) 62 (49; 78) | (N=124) 10212 (8419; 12388) | (N=126) 442 (377; 518) | (N=123) 436 (375; 506) | |
| 12-17 years | EBL2002 | (N=53) <LLOQ (<LLOQ; 37) | (N=53) 13532 (10732; 17061) | (N=41) 577 (454; 734) | (N=52) 541 (433; 678) |
| EBL3001 | (N=142) 65 (52; 81) | (N=134) 9929 (8172; 12064) | (N=135) 469 (397; 554) | (N=132) 386 (326; 457) |
Data shown for vaccinated participants who received the 2-dose vaccine regimen in the Per Protocol Analysis Set.
EU = ELISA Units
CI = Confidence interval
N = Number of participants with data
LLOQ = Lower limit of quantification
Immunogenicity data in adults after Zabdeno booster vaccination
The immune response to a booster vaccination of Zabdeno administered 1 or 2 years after the primary vaccination regimen was evaluated in 2 clinical studies (see Table 5). Booster vaccination resulted in the rapid activation of an anamnestic response, with a 40- to 56-fold increase in antibody concentrations within 7 days. The magnitude of the response in terms of fold-increase and post-booster GMC was similar irrespective of the time since primary vaccination (1 or 2 years).
Table 5. EBOV GP-specific Binding Antibody Responses to Zabdeno Booster Vaccination in Adults: GMC EU/mL (95% CI):
| Study | Pre-booster | 7 days post-booster | 21 days post-booster | 1 year post-booster |
|---|---|---|---|---|
| EBL2002^a^ | (N=39) 366 (273; 491) | (N=39) 20416 (15432; 27009) | (N=39) 41643 (32045; 54116) | (N=37) 4383 (2969; 6470) |
| EBL3001^b^ | (N=29) 274 (193; 387) | (N=25) 11166 (5881; 21201) | (N=29) 30411 (21972; 42091) | (N=26) 3237 (2305; 4547) |
a booster vaccination administered 1 year after primary vaccination
b booster vaccination administered 2 years after primary vaccination
Data shown for vaccinated participants who received the booster vaccination in the Per Protocol Analysis Set.
EU = ELISA Units
CI = Confidence interval
N = Number of participants with data
Long term persistence of antibodies in adults
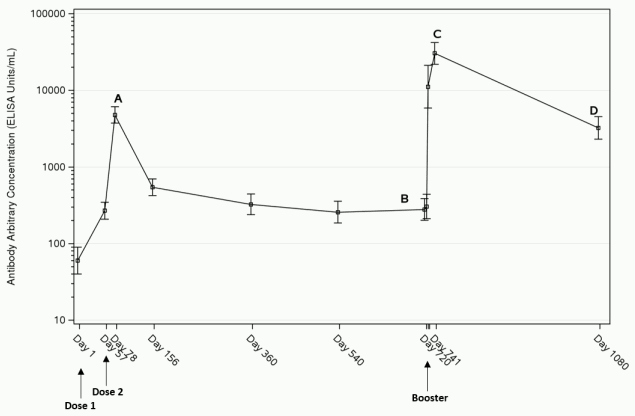
Three weeks after completion of the 2-dose primary vaccination regimen, the immune response (GMC) reaches its peak (“A” in figure 1 below). After the peak the response declines by 6 months and remains stable at least 1 year post-dose 1 (Table 3). As illustrated by the data on 43 adults in study EBL3001, the response remains stable also at two years post-dose 1 (latest time point available) (“B” in figure 1 below). After administration of a booster dose of Zabdeno, a rapid anamnestic response is observed within 7 days. The highest binding antibody concentrations are observed 21 days post-booster dose (“C” in figure 1 below), followed by a decline in antibody concentrations. At 1 year post-booster dose, GMCs were higher than before administration of the booster dose (“D” in figure 1 below).
Figure 1. EBOV GP-specific Binding Antibody Responses after the Zabdeno, Mvabea 2-dose vaccine regimen and Zabdeno booster vaccination 2 years after the primary vaccination regimen in adults in study EBL3001a; GMC (95% CI):
a The analysis is based on the per protocol analysis set. The error bars represent the Geometric Mean Concentration and its 95% confidence interval.
The European Medicines Agency has deferred the obligation to submit the results of studies with Mvabea for the prevention of Ebola virus disease in one or more subsets of the paediatric population (see section 4.2 for information on paediatric use).
This vaccine has been authorised under ‘exceptional circumstances’. This means that for scientific reasons it has been impossible to get complete information on this vaccine. The European Medicines Agency will review any new information which may become available every year and this SmPC will be updated as necessary.
5.2. Pharmacokinetic properties
Not applicable.
5.3. Preclinical safety data
Non-clinical data revealed no special hazard for humans based on repeated dose toxicity and local tolerance studies, and a reproductive toxicity study in rabbits.
General (repeated dose) toxicity studies, including local tolerance
Vaccination of rabbits with various Zabdeno and Mvabea vaccine regimens was well tolerated when administered intramuscularly at full human dose levels. The vaccine-related findings (reflected by inflammatory changes at the injection site, increases in fibrinogen, C-reactive protein and globulin, and microscopic findings of increased lymphoid cellularity and/or germinal centres in the draining lymph nodes and spleen) were noted to be recovering 2 weeks after the last vaccination, and reflect a normal, physiological response associated with vaccination. There were no effects noted that were considered to be adverse.
Fertility / Reproductive and Developmental Toxicity
Biodistribution studies conducted in the rabbit did not show distribution of the MVA-BN vector to the gonads (testes, ovaries) following IM injection.
The general (repeated dose) toxicity studies with Zabdeno and Mvabea vaccine regimens have not revealed any effects on male sex organs that would impair male fertility. In addition, the general and/or reproductive toxicity studies did not reveal any evidence of impaired female fertility. In a reproductive toxicity study, Zabdeno and Mvabea vaccine regimens did not induce maternal or developmental toxicity following maternal exposure during the premating and gestation period. In this study, the vaccine regimens elicited detectable EBOV GP-specific maternal antibody titres that were transferred to the foetuses.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.