NEMLUVIO Powder and solvent for solution for injection Ref.[114712] Active ingredients: Nemolizumab
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: Galderma International, La Defense 4, Tour Europlaza, 20 Avenue Andre Prothin, 92927 Paris La Defense Cedex, France
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Other dermatological preparations, agents for dermatitis, excluding corticosteroids
ATC code: D11AH12
Mechanism of action
Nemolizumab is a humanised IgG2 monoclonal antibody that inhibits interleukin-31 (IL-31) signalling by binding selectively to interleukin-31 receptor alpha (IL-31 RA). IL-31 is a naturally occurring cytokine that is involved in pruritus, inflammation, epidermal dysregulation, and fibrosis.
Nemolizumab inhibited IL-31-induced responses including the release of proinflammatory cytokines and chemokines.
In atopic dermatitis clinical studies, nemolizumab was found to modulate gene expression related to the pathophysiology of atopic dermatitis, with a primary impact on immune system processes, by decreasing the inflammatory and proliferative profile of specific immune cells (T-cells and monocytes/macrophages) without leading to immunosuppression.
In prurigo nodularis clinical studies, nemolizumab was found to modulate molecular processes related to the pathophysiology of prurigo nodularis, with impact on pruritus, inflammation, epidermal differentiation and fibrosis.
Pharmacodynamic effect
Immunogenicity
Anti-drug antibodies (ADA) were very commonly detected. No evidence of ADA impact on pharmacokinetics, efficacy or safety was observed.
Clinical efficacy and safety in atopic dermatitis
Adults and adolescents with atopic dermatitis
The efficacy and safety of nemolizumab with concomitant topical background therapy was evaluated in two randomised, double-blind, placebo-controlled pivotal studies (ARCADIA 1 and ARCADIA 2) that enrolled a total of 1728 subjects 12 years of age and older with moderate-to-severe atopic dermatitis not adequately controlled by topical treatments. Disease severity was defined by an Investigator’s Global Assessment (IGA) score of 3 (moderate) and 4 (severe) in the overall assessment of atopic dermatitis, an Eczema Area and Severity Index (EASI) score of ≥16, a minimum body surface area (BSA) involvement of ≥10%, and a Peak Pruritus Numeric Rating Scale (PP NRS) score of ≥4.
Subjects in the studies received initial subcutaneous injections of either nemolizumab 60 mg, followed by 30 mg injections every 4 weeks (Q4W), or matching placebo. Concomitant low and/ or medium potency TCS and/or TCI were administered both in nemolizumab and placebo groups for at least 14 days prior to baseline and continued during the study. Based on disease activity, these concomitant therapies could be tapered and/or discontinued at investigator discretion.
After 16 weeks, subjects achieving either EASI-75 or IGA success continued into the study maintenance period for another 32 weeks to evaluate the maintenance of response achieved at Week 16. Nemolizumab responders were re-randomised to either nemolizumab 30 mg every 4 weeks, nemolizumab 30 mg every 8 weeks or placebo every 4 weeks (all groups continued background TCS/TCI). Subjects randomised to placebo in the initial treatment period who achieved the same clinical response at Week 16 continued to receive placebo every 4 weeks. Non-responders at Week 16, subjects who lost clinical response during the maintenance period and subjects who completed maintenance period had the opportunity to enrol into the open-label study (ARCADIA LTE) and receive treatment with nemolizumab 30 mg every 4 weeks up to 200 weeks.
Endpoints
Both ARCADIA 1 and ARCADIA 2 assessed the primary endpoints of:
- Proportion of subjects with an IGA success (defined as an IGA of 0 [clear] or 1 [almost clear] and a ≥2-point reduction from baseline) at Week 16
- Proportion of subjects with EASI-75 (≥75% improvement in EASI from baseline) at Week 16
Key secondary endpoints included PP NRS improvement ≥4 from baseline at Weeks 1, 2, 4 and 16, PP NRS <2 at Week 4 and Week 16, Sleep Disturbance Numeric Rating Scale (SD NRS) improvement ≥4 from baseline at Week 16, subjects with both EASI-75 and PP NRS improvement ≥4 from baseline at Week 16, and subjects with both IGA success and PP NRS improvement ≥4 from baseline at Week 16.
Baseline characteristics
In these studies, at baseline, 51.0% of subjects were male, 79.9% were White, and the mean weight was 75.0 kg. The mean age was 34.1 years, 15.4% of subjects were adolescents (12-17 years) and 5.3% were 65 years of age or older. 70% of subjects had a baseline IGA score of 3 (moderate AD), and 30% of subjects had a baseline IGA score of 4 (severe AD). The mean (SD) baseline EASI score was 27.5 (10.5), the baseline weekly average (SD) PP NRS was 7.1 (1.5) (severe itch) and baseline weekly average (SD) SD NRS was 5.8 (2.2). Overall, 63.3% of subjects received other previous systemic treatments for atopic dermatitis.
Clinical response
ARCADIA 1 and ARCADIA 2 – Adults and Adolescents – induction period, Week 0 to Week 16
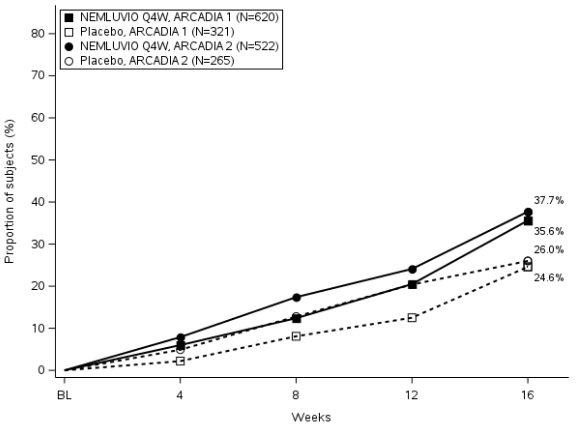
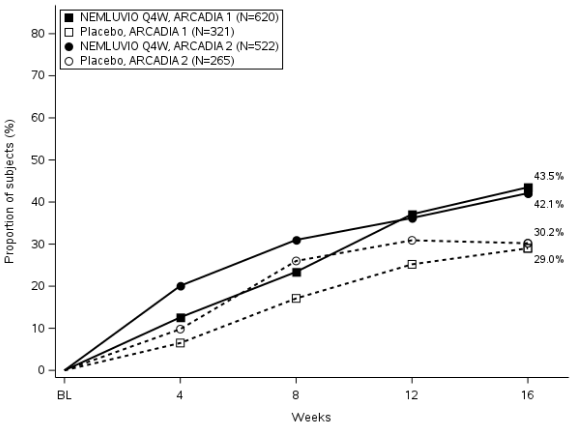
Nemolizumab was statistically significantly superior to placebo with respect to skin-related co-primary endpoints IGA success and EASI-75 over 16 weeks (Table 2). Results for both co-primary endpoints were consistent in the severe pruritus population (baseline PP NRS ≥7).
Table 2. Efficacy Results of nemolizumab (30 mg Q4W) with concomitant TCS/TCI in ARCADIA 1 and ARCADIA 2 at Week 16:
| ARCADIA 1 | ARCADIA 2 | |||
|---|---|---|---|---|
| Nemolizumab + TCS/TCI | Placebo + TCS/TCI | Nemolizumab + TCS/TCI | Placebo + TCS/TCI | |
| Number of subjects randomised and dosed (Baseline PP NRS ≥4) | 620 | 321 | 522 | 265 |
| % of subjects with IGA 0 or 1a | 35.6# | 24.6 | 37.7# | 26.0 |
| % of subjects with EASI-75a | 43.5* | 29.0 | 42.1# | 30.2 |
a Subjects who received rescue treatment or with missing data were considered as non-responders
* p-value <0.0001, #p-value <0.001
Strata adjusted p-value is based on the CMH test stratified by PP NRS and IGA score at baseline.
Figure 1. Proportion of subjects with IGA success and EASI-75 from baseline to Week 16 in ARCADIA 1 and ARCADIA 2:
Figure 1a. IGA Success
Figure 1b. EASI-75
Significant improvement in pruritus for subjects treated with nemolizumab in ARCADIA 1 and ARCADIA 2 compared to placebo based on PP NRS improvements ≥4 and PP NRS percent change from baseline was observed starting at Week 1 and was maintained up to Week 16 (Table 3 and Figure 2). Results were consistent in the severe pruritus population (baseline PP NRS ≥7).
Table 3. Efficacy results on itch for nemolizumab with concomitant TCS/TCI in ARCADIA 1 and ARCADIA 2 up to Week 16:
| ARCADIA 1 | ARCADIA 2 | |||
|---|---|---|---|---|
| Nemolizumab + TCS/TCI | Placebo + TCS/TCI | Nemolizumab + TCS/TCI | Placebo + TCS/TCI | |
| Number of subjects randomised and dosed (Baseline PP NRS ≥4)a | 620 | 321 | 522 | 265 |
| % of subjects with PP NRS improvement ≥4a | ||||
| At Week 1 | 4.7§ | 1.2 | 6.7* | 0.4 |
| At Week 2 | 17.7* | 3.1 | 16.9* | 1.9 |
| At Week 4 | 27.4* | 6.5 | 26.1* | 5.3 |
| At Week 16 | 42.7* | 17.8 | 41.0* | 18.1 |
| % of subjects with PP NRS <2a | ||||
| At Week 4 | 16.0* | 3.7 | 15.9* | 2.6 |
| At Week 16 | 30.6* | 11.2 | 28.4* | 11.3 |
| Mean change from baseline (%) | ||||
| At Week 16 | -56.1* | -30.6 | -55.6* | -30.3 |
a Subjects who received rescue treatment or with missing data were considered as non-responders
* p-value < 0.0001, §p-value < 0.05
Strata adjusted p-value is based on the CMH test stratified by PP NRS and IGA score at baseline
Figure 2. Proportion of subject with PP NRS improvement of ≥4 from baseline up to Week 16 in ARCADIA 1 and ARCADIA 2:
In patients with a body weight ≥90kg, in a post-hoc analysis in each of the pivotal studies there was no difference in anti-inflammatory response (IGA 0 or 1 and EASI 75) at Week 16 between nemolizumab and placebo arms, though the effect was observed in reducing pruritus (PP NRS).
The Sleep Disturbance Numeric Rating Scale (SD NRS) is a daily scale used by the subjects to report the degree of their sleep loss related to atopic dermatitis. A significant improvement in sleep disturbance was observed at Week 16 when compared to placebo (Table 4). Results were consistent in the severe pruritus population (baseline PP NRS ≥7).
Table 4. Efficacy on Sleep Disturbance for nemolizumab with concomitant TCS/TCI in ARCADIA 1 and ARCADIA 2 at Week 16:
| ARCADIA 1 | ARCADIA 2 | |||
|---|---|---|---|---|
| Nemolizumab + TCS/TCI | Placebo + TCS/TCI | Nemolizumab + TCS/TCI | Placebo + TCS/TCI | |
| Number of subjects randomised and dosed (Baseline PP NRS ≥4)a | 620 | 321 | 522 | 265 |
| % of subjects with SD NRS improvement ≥4a Mean change from baseline (%) | 37.9* -64.6 | 19.9 -38.1 | 33.5* -59.7 | 16.2 -35.4 |
a Subjects who received rescue treatment or with missing data were considered as non-responders
* p-value <0.0001
Strata adjusted p-value is based on the CMH test stratified by PP NRS and IGA score at baseline
Adolescents with atopic dermatitis (12 to 17 years of age)
The efficacy results of the ARCADIA 1, ARCADIA 2 studies at Week 16 for paediatric subjects 12 to 17 years of age are presented in Table 5. The results in the paediatric subject population were generally consistent with the results in the adult subject population. Results in co-primary and key secondary endpoints were consistent in the severe pruritus population (baseline PP NRS ≥7).
Table 5. Efficacy Results for nemolizumab (30 mg Q4W) with concomitant TCS/TCI in ARCADIA 1 and ARCADIA 2 at Week 16 in paediatric subjects 12 to 17 years of age:
| ARCADIA 1 AND ARCADIA 2 | ||
|---|---|---|
| Nemolizumab + TCS/TCI | Nemolizumab + TCS/TCI | |
| Number of subjects randomised and dosed (Baseline PP NRS ≥4) | 179 | 90 |
| % of subjects with IGA 0 or 1a | 48.9* | 34.4 |
| % of subjects with EASI-75a | 53.4§ | 43.3 |
| % of subjects with PP-NRS improvement ≥4a | 40.9# | 17.8 |
| % of subjects with PP NRS <2a | 30.1≠ | 6.7 |
| % of subjects with SD NRS improvement ≥4a | 31.8∞ | 20.0 |
a Subjects who received rescue treatment or with missing data were considered as non-responders
≠ p-value < 0.0001, #p-value < 0.001, *p-value < 0.05, ∞p-value = 0.0591, §p-value = 0.1824
Strata adjusted p-value is based on the CMH test stratified by PP NRS and IGA score at baseline
ARCADIA 1 and ARCADIA 2 – Adults and Adolescents – maintenance period, Week 16 to Week 48
The clinical response in nemolizumab responders (IGA 0/1 or EASI-75 at Week 16) was evaluated between Week 16 and Week 48 in ARCADIA 1 and ARCADIA 2 studies. For the maintenance treatment period, 507 nemolizumab responders were re-randomised to nemolizumab 30 mg Q4W, nemolizumab 30 mg Q8W or placebo Q4W (nemolizumab withdrawal) with concomitant TCS/TCI. The pooled efficacy results with descriptive analysis only for this period in the pivotal studies (ARCADIA 1 and ARCADIA 2) with nemolizumab at Week 48 are presented in Table 6.
Table 6. Maintenance Period Pooled Efficacy Results for nemolizumab with concomitant TCS/TCI in ARCADIA 1 and ARCADIA 2 at Week 48:
| Nemolizumab + TCS/TCI Q4W N=169 | Nemolizumab + TCS/TCI Q8W N=169 | Placebo + TCS/TCI Q4W (Nemolizumab withdrawal) N=169 | |
|---|---|---|---|
| % of subjects with IGA 0 or 1a | |||
| Week 16 (maintenance baseline) | 84.0 | 84.0 | 77.5 |
| Week 48 Strata-adjusted proportion difference () Strata-adjusted 95 CI | 61.5 11.8 (1.3, 22.3) | 60.4 10.7 (0.3, 21.0) | 49.7 |
| % of subjects with EASI-75a (95% CI) | |||
| Week 16 (maintenance/baseline) | 96.4 | 96.4 | 92.9 |
| Week 48 Strata-adjusted proportion difference () Strata-adjusted 95 CI | 76.3 12.4 (2.7, 22.0) | 75.7 11.8 (2.1, 21.5) | 63.9 |
a Subjects who received rescue treatment or with missing data were considered as non-responders
Clinical efficacy and safety in adults with prurigo nodularis
The efficacy and safety of nemolizumab as monotherapy was evaluated in two randomised, double-blind, placebo-controlled pivotal studies (OLYMPIA 1 and OLYMPIA 2) that enrolled a total of 560 subjects 18 years of age and older with moderate-to-severe prurigo nodularis. Disease severity was defined using an Investigator’s Global Assessment (IGA) in the overall assessment of prurigo nodularis nodules on a severity scale of 0 to 4. Subjects enrolled in these two studies had an IGA score ≥3, severe pruritus as defined by a weekly average of the peak pruritus numeric rating scale (PP-NRS) score of ≥7 on a scale of 0 to 10, and greater than or equal to 20 nodular lesions. OLYMPIA 1 and OLYMPIA 2 assessed the effect of nemolizumab monotherapy on the signs and symptoms of prurigo nodularis, targeting improvement in skin lesions and pruritus over 16 weeks. OLYMPIA 1 had a 24-week treatment period and OLYMPIA 2 a 16-week treatment period.
In the nemolizumab treatment group, subjects weighing less than 90 kg received subcutaneous injections of nemolizumab 60 mg (2 injections of 30 mg) at Week 0, followed by 30 mg injections every 4 weeks, and subjects weighing 90 kg or more received subcutaneous injections of nemolizumab 60 mg (2 injections of 30 mg) at Week 0 and every 4 weeks.
Endpoints
Both OLYMPIA 1 and OLYMPIA 2 assessed the same two primary endpoints:
- Proportion of subjects with an improvement of ≥4 from baseline in Peak Pruritus Numeric Rating Scale (PP NRS) at Week 16
- Proportion of subjects with an IGA success (defined as an IGA of 0 [Clear] or 1 [Almost Clear], and a ≥2-point improvement from baseline) at Week 16
Key secondary endpoints included PP NRS improvement ≥4 from baseline at Week 4, PP NRS <2 at Week 4 and Week 16, Sleep Disturbance Numeric Rating Scale (SD NRS) improvement ≥4 from baseline at Week 4 and 16.
Baseline characteristics
In these studies, at baseline, 59.6% of subjects were female, 81.4% were white, the mean weight was 82.6 kg, the mean age was 55.2 years and 25.4% of subjects were older than 65 years of age. The baseline weekly average PP NRS score was a mean (SD) of 8.4 (0.9). Fifty-eight (58) % of subjects had a baseline IGA score of 3 (moderate PN) and 42% of subjects had a baseline IGA of 4 (severe PN).
Clinical response
Pivotal studies (OLYMPIA 1 and OLYMPIA 2) – Week 0 to Week 16
Results of the pivotal studies evaluating treatment of nemolizumab in OLYMPIA 1 and OLYMPIA 2 are presented in Table 7 and show significant improvement in nemolizumab treated subjects, compared to placebo for both primary endpoints (Figure 3 and Figure 4).
Table 7. Efficacy Results for nemolizumab monotherapy (Q4W) in OLYMPIA 1 and OLYMPIA 2:
| OLYMPIA 1 | OLYMPIA 2 | |||
|---|---|---|---|---|
| Nemolizumab | Placebo | Nemolizumab | Placebo | |
| Number of subjects randomised | 190 | 96 | 183 | 91 |
| % of subjects with improvement of PP NRS ≥4 from baselinea | ||||
| Week 4 | 41.1* | 6.3 | 41.0* | 7.7 |
| Week 16 | 58.4* | 16.7 | 56.3* | 20.9 |
| % of subjects with IGA 0 or 1 at Week 16a | 26.3# | 7.3 | 37.7* | 11 |
| % of subjects with PP NRS <2a | ||||
| Week 4 | 21.6* | 1.0 | 19.7* | 2.2 |
| Week 16 | 34.2* | 4.2 | 35.0* | 7.7 |
| % of subjects with improvement of SD NRS ≥4 from baselinea | ||||
| Week 4 | 31.1* | 5.2 | 37.2* | 9.9 |
| Week 16 | 50.0* | 11.5 | 51.9* | 20.9 |
a If a subject received any rescue therapy, composite variable strategy is applied, the underlying data at/after receipt of rescue therapy is set as worst possible value, and the response is derived from underlying data value. Subjects with missing results are considered as non-responders.
* p-value < 0.0001, #p-value = 0.0025 Strata adjusted using the randomised stratification variables (analysis centre and baseline body weight (<90 kg, ≥90 kg)
Figure 3. Proportion of Subjects with PP-NRS Improvement ≥4 from baseline to Week 16:
Figure 4. Proportion of IGA responders from baseline to Week 16:
5.2. Pharmacokinetic properties
Absorption
Following an initial subcutaneous dose of 60 mg in patients with AD or PN, the population PK estimated mean (SD) peak concentration (Cmax) was 6.7 (2.20) μg/mL by approximately 6 days post dose.
Following multiple doses in subjects with atopic dermatitis, the population PK estimated mean (SD) steady-state trough concentrations of nemolizumab were 2.63 (1.27) μg/mL for 30 mg administered Q4W and 0.74 (0.44) μg/mL for 30 mg administered Q8W.
Following multiple doses in subjects with prurigo nodularis, the population PK estimated mean (SD) steady-state trough concentrations of nemolizumab 3.04 (1.23) μg/mL in patients with body weight <90 kg for 30 mg administered Q4W; and 3.66 (1.63) μg/mL in patients with body weight ≥90 kg for 60 mg administered Q4W.
In both atopic dermatitis and prurigo nodularis population, steady state concentrations of nemolizumab were achieved by week 4 after a 60 mg loading dose and by week 12 without a loading dose.
A loading dose is proposed for subjects with PN with body weight <90 kg. However, for subjects with body weight ≥90 kg no loading dose is proposed because the 60 mg dose was sufficient to achieve similar steady-state concentrations of nemolizumab as the 30 mg dose (with 60 mg loading dose) after the second dose (at Week 8).
Distribution
Based on a population PK analysis, the apparent volume of distribution (V/F) was 7.67 L.
Biotransformation
Specific metabolism studies were not conducted because nemolizumab is a protein. Nemolizumab is expected to be metabolised into small peptides by catabolic pathways.
Elimination
Nemolizumab is expected to be degraded in the same manner as endogenous IgG. In the population PK analysis, the terminal elimination half-life (SD) of nemolizumab was estimated to be 18.9 (4.96) days and apparent systemic clearance (Cl/F) was estimated to be 0.26 L/day.
Linearity/non-linearity
After a single dose, nemolizumab exhibited linear pharmacokinetics with exposures increasing in dose-proportional manner between 0.03 and 3 mg/kg. After multiple doses, nemolizumab systemic exposure increased in an approximately dose-proportional manner across the SC dose range up to 30 mg. There was a slight decrease in bioavailability by 9% with the 60 mg SC dose.
Special populations
Gender, age and ethnicity
Gender, age (range 12 to 85 years for AD, and 18 to 84 years for PN), and ethnicity did not have a clinically relevant effect on the pharmacokinetics of nemolizumab.
Hepatic impairment
Nemolizumab, as a monoclonal antibody, is not expected to undergo significant hepatic elimination. No clinical studies have been conducted to evaluate the effect of hepatic impairment on the pharmacokinetics of nemolizumab. Mild to moderate hepatic impairment was not found to affect the PK of nemolizumab determined by population PK analysis. No data are available in patients with severe hepatic impairment.
Renal impairment
Nemolizumab, as a monoclonal antibody, is not expected to undergo significant renal elimination. No clinical studies have been conducted to evaluate the effect of renal impairment on the pharmacokinetics of nemolizumab. Population PK analysis did not identify mild or moderate renal impairment as having a clinically meaningful influence on the systemic exposure of nemolizumab. Very limited data are available in patients with severe renal impairment.
Body weight
Nemolizumab exposure was lower in subjects with higher body weight.
Atopic dermatitis:
The difference in systemic exposure due to body weight had no clinically meaningful impact on efficacy. Dose adjustment based on body weight is not needed (see section 4.2).
Prurigo nodularis:
The variability in systemic exposure due to body weight had a clinically meaningful impact on skin lesion efficacy as assessed by IGA response but not on pruritus improvement and does require dose adjustment in subjects with PN (see section 4.2).
Paediatric population
Atopic dermatitis:
In the population PK analysis, no clinically relevant difference in the pharmacokinetics of nemolizumab was estimated in paediatric subjects 12-17 years of age compared to adults. Dose adjustment in this population is not recommended.
5.3. Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology and repeated dose toxicity.
The mutagenic potential of nemolizumab has not been evaluated; however monoclonal antibodies are not expected to alter DNA or chromosomes.
Carcinogenicity studies have not been conducted with nemolizumab. Evaluation of the available evidence related to IL-31 inhibition and animal toxicology data does not suggest carcinogenic potential.
No effects on fertility parameters were observed in sexually mature cynomolgous monkeys after a long-term subcutaneous treatment with nemolizumab. In the group of dams treated with 25 mg/kg of nemolizumab every two weeks from early organogenesis to delivery, a slight increase in the incidence of offspring death was observed during the early postnatal period. The dams exposures (AUC) were 43- or 34-fold higher than human exposure at maximum recommended human dose in AD or PN patients respectively. A relation of this finding to nemolizumab cannot be excluded.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.