NILEMDO Film-coated tablet Ref.[27925] Active ingredients: Bempedoic acid
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Daiichi Sankyo Europe GmbH, Zielstattstrasse 48, 81379 Munich, Germany
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Lipid modifying agents, other lipid modifying agents
ATC code: C10AX15
Mechanism of action
Bempedoic acid is an adenosine triphosphate citrate lyase (ACL) inhibitor that lowers low-density lipoprotein cholesterol (LDL-C) by inhibition of cholesterol synthesis in the liver. ACL is an enzyme upstream of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase in the cholesterol biosynthesis pathway. Bempedoic acid requires coenzyme A (CoA) activation by very long-chain acyl-CoA synthetase 1 (ACSVL1) to ETC-1002-CoA. ACSVL1 is expressed primarily in the liver and not in skeletal muscle. Inhibition of ACL by ETC-1002-CoA results in decreased cholesterol synthesis in the liver and lowers LDL-C in blood via upregulation of low-density lipoprotein receptors. Additionally, inhibition of ACL by ETC-1002-CoA results in concomitant suppression of hepatic fatty acid biosynthesis.
Pharmacodynamic effects
Administration of bempedoic acid alone and in combination with other lipid modifying medicinal products decreases LDL-C, non-high density lipoprotein cholesterol (non-HDL-C), apolipoprotein B (apo B), total cholesterol (TC), and C-reactive protein (CRP) in patients with hypercholesterolaemia or mixed dyslipidaemia.
Because patients with diabetes are at elevated risk for atherosclerotic cardiovascular disease, the clinical trials of bempedoic acid included patients with diabetes mellitus. Among the subset of patients with diabetes, lower levels of HbA1c were observed as compared to placebo (on average 0.2%). In patients without diabetes, no difference in HbA1c was observed between bempedoic acid and placebo and there were no differences in the rates of hypoglycaemia.
Cardiac electrophysiology
At a dose of 240 mg (1.3 times the approved recommended dose), bempedoic acid does not prolong the QT interval to any clinically relevant extent.
Clinical efficacy and safety
Clinical efficacy and safety in primary hypercholesterolaemia and mixed dyslipidaemia
The efficacy of Nilemdo was investigated in four multi-centre, randomised, double-blind, placebocontrolled phase 3 primary hyperlipidaemia studies involving 3 623 adult patients with hypercholesterolaemia or mixed dyslipidaemia, with 2 425 patients randomised to bempedoic acid. All patients received bempedoic acid 180 mg or placebo orally once daily. In two trials, patients were taking background lipid-modifying therapies consisting of a maximum tolerated dose of statin, with or without other lipid-modifying therapies. Two trials were conducted in patients with documented statin intolerance. The primary efficacy endpoint in all Phase 3 trials was the mean percent reduction from baseline in LDL-C at week 12 as compared with placebo.
Combination therapy with statins
CLEAR Wisdom (Study 1002-047) was a multi-centre, randomised, double-blind, placebo-controlled, 52-week phase 3 primary hyperlipidaemia study in patients with hypercholesterolaemia or mixed dyslipidaemia. Efficacy of Nilemdo was evaluated at week 12. The trial included 779 patients randomised 2:1 to receive either bempedoic acid (n=522) or placebo (n=257) as add-on to a maximum tolerated lipid lowering therapy. Maximum tolerated lipid lowering therapy was defined as a maximum tolerated statin dose (including statin regimens other than daily dosing and no to very low doses) alone or in combination with other lipid-lowering therapies. Patients on simvastatin 40 mg/day or higher were excluded from the trial.
Overall, the mean age at baseline was 64 years (range: 28 to 91 years), 51% were ≥65 years old, 36% were women, 94% were White, 5% were Black, and 1% were Asian. The mean baseline LDL-C was 3.1 mmol/L (120.4 mg/dL). At the time of randomisation, 91% of patients were receiving statin therapy and 53% were receiving high-intensity statin therapy. Bempedoic acid significantly reduced LDL-C from baseline to week 12 compared with placebo (p<0.001). Bempedoic acid also significantly reduced non-HDL-C, apo B, and TC.
CLEAR Harmony (Study 1002-040) was a multi-centre, randomised, double-blind, placebo-controlled 52-week phase 3 primary hyperlipidaemia study evaluating safety and efficacy of bempedoic acid in patients with hypercholesterolaemia or mixed dyslipidaemia. Efficacy of Nilemdo was evaluated at week 12. The trial included 2 230 patients randomised 2:1 to receive either bempedoic acid (n=1 488) or placebo (n=742) as add-on to a maximum tolerated lipid lowering therapy. Maximum tolerated lipid lowering therapy was defined as a maximum tolerated statin dose (including statin regimens other than daily dosing and very low doses) alone or in combination with other lipid lowering therapies. Patients on simvastatin 40 mg per day or higher and patients on PCSK9 inhibitors were excluded from the trial.
Overall, the mean age at baseline was 66 years (range: 24 to 88 years), 61% were ≥65 years old, 27% were women, 96% were White, 3% were Black, and 1% were Asian. The mean baseline LDL-C was 2.7 mmol/L (103.2 mg/dL). At the time of randomisation, all patients were receiving statin therapy and 50% were receiving high-intensity statin therapy. Bempedoic acid significantly reduced LDL-C from baseline to week 12 compared with placebo (p<0.001). A significantly higher proportion of patients achieved an LDL-C of ˂1.81 mmol/L (˂70 mg/dL) in the bempedoic acid group as compared with placebo at week 12 (32% versus 9%, p<0.001), bempedoic acid also significantly reduced non-HDL-C, apo B, and TC (see table 2).
Table 2. Treatment effects of Nilemdo compared with placebo in patients with primary hypercholesterolaemia or mixed dyslipidaemia – mean percent change from baseline to week 12:
| CLEAR Wisdom (Study 1002-047) (N=779) | CLEAR Harmony (Study 1002-040) (N=2 230) | |||
|---|---|---|---|---|
| Nilemdo n=522 | Placebo n=257 | Nilemdo n=1 488 | Placebo n=742 | |
| LDL-Ca, n | 498 | 253 | 1 488 | 742 |
| LS Mean | -15.1 | 2.4 | -16.5 | 1.6 |
| non-HDL-Ca, n | 498 | 253 | 1 488 | 742 |
| LS Mean | -10.8 | 2.3 | -11.9 | 1.5 |
| apo Ba, n | 479 | 245 | 1 485 | 736 |
| LS Mean | -9.3 | 3.7 | -8.6 | 3.3 |
| TCa, n | 499 | 253 | 1 488 | 742 |
| LS Mean | -9.9 | 1.3 | -10.3 | 0.8 |
apo B=apolipoprotein B; HDL-C=high-density lipoprotein cholesterol; LDL C=low-density lipoprotein cholesterol; LS=least squares; TC=total cholesterol.
Background statin (1002-047): atorvastatin, simvastatin, rosuvastatin, pravastatin, fluvastatin, pitavastatin, and lovastatin.
Background statin (1002-040): atorvastatin, simvastatin, pravastatin.
a Percent change from baseline was analysed using analysis of covariance (ANCOVA), with treatment and randomisation strata as factors and baseline lipid parameter as a covariate.
Statin intolerant patients
CLEAR Tranquility (Study 1002-048) was a multi-centre, randomised, double-blind, placebocontrolled 12-week phase 3 primary hyperlipidaemia study evaluating the efficacy of Nilemdo versus placebo in lowering LDL-C when added to ezetimibe in patients with elevated LDL-C who had a history of statin intolerance and were unable to tolerate more than the lowest approved starting dose of a statin. The trial included 269 patients randomised 2:1 to receive either bempedoic acid (n=181) or placebo (n=88) as add-on to ezetimibe 10 mg daily for 12 weeks.
Overall, the mean age at baseline was 64 years (range: 30 to 86 years), 55% were ≥65 years old, 61% were women, 89% were White, 8% were Black, 2% were Asian, and 1% were other. The mean baseline LDL-C was 3.3 mmol/L (127.6 mg/dL). At the time of randomisation, 33% of patients on bempedoic acid versus 28% on placebo were receiving statin therapy at less than or equal to lowest approved doses. Bempedoic acid significantly reduced LDL-C from baseline to week 12 compared with placebo (p<0.001). Bempedoic acid also significantly reduced non-HDL-C, apo B, and TC (see table 3).
CLEAR Serenity (Study 1002-046) was a multi-centre, randomised, double-blind, placebo-controlled 24-week phase 3 primary hyperlipidaemia study evaluating the efficacy of Nilemdo versus placebo in patients with elevated LDL-C who were statin-intolerant or unable to tolerate two or more statins, one at the lowest dose. Patients able to tolerate a dose that was less than the approved starting dose of a statin were allowed to stay on that dose during the study. Efficacy of bempedoic acid was evaluated at week 12. The trial included 345 patients randomised 2:1 to receive either bempedoic acid (n=234) or placebo (n=111) for 24 weeks. At the time of randomisation, 8% of patients on bempedoic acid versus 10% on placebo were receiving statin therapy at less than the lowest approved doses and 36% of patients on bempedoic acid versus 30% of patients on placebo were on other nonstatin lipid-modifying therapies.
Overall, the mean age at baseline was 65 years (range: 26 to 88 years), 58% were ≥65 years old, 56% were women, 89% were White, 8% were Black, 2% were Asian, and 1% were other. The mean baseline LDL-C was 4.1 mmol/L (157.6 mg/dL).
Bempedoic acid significantly reduced LDL-C from baseline to week 12 compared with placebo (p<0.001). Bempedoic acid also significantly reduced non-HDL-C, apo B, and TC (see table 3).
Treatment in the absence of lipid-modifying therapies
In CLEAR Serenity (Study 1002-046), 133 patients in the bempedoic acid group and 67 patients in the placebo group were on no background lipid-modifying therapies. Bempedoic acid significantly reduced LDL-C from baseline to week 12 compared with placebo in this subgroup. The difference between bempedoic acid and placebo in mean percent change in LDL-C from baseline to week 12 was -22.1% (CI: -26.8%, -17.4%; p<0.001).
Table 3. Treatment effects of Nilemdo compared with placebo in statin intolerant patients – mean percent change from baseline to week 12:
| CLEAR Tranquility (Study 1002-048) (N=269) | CLEAR Serenity (Study 1002-046) (N=345) | |||
|---|---|---|---|---|
| Nilemdo n=181 | Placebo n=88 | Nilemdo n=234 | Placebo n=111 | |
| LDL-Ca, n | 175 | 82 | 224 | 107 |
| LS Mean | -23.5 | 5.0 | -22.6 | -1.2 |
| non-HDL-Ca, n | 175 | 82 | 224 | 107 |
| LS Mean | -18.4 | 5.2 | -18.1 | -0.1 |
| apo Ba, n | 174 | 81 | 218 | 104 |
| LS Mean | -14.6 | 4.7 | -14.7 | 0.3 |
| TCa, n | 176 | 82 | 224 | 107 |
| LS Mean | -15.1 | 2.9 | -15.4 | -0.6 |
apo B=apolipoprotein B; HDL-C=high-density lipoprotein cholesterol; LDL C=low-density lipoprotein cholesterol; LS=least squares; TC=total cholesterol.
Background statin (1002-048): atorvastatin, simvastatin, rosuvastatin, pravastatin, lovastatin
Background statin (1002-046): atorvastatin, simvastatin, pitavastatin, rosuvastatin, pravastatin, lovastatin
a Percent change from baseline was analysed using analysis of covariance (ANCOVA), with treatment and randomisation strata as factors and baseline lipid parameter as a covariate.
In all four trials, the maximum LDL-C lowering effects were observed as early as week 4 and efficacy was maintained throughout the trials. These results were consistent across all subgroups studied in any of the trials, including age, gender, race, ethnicity, region, history of diabetes, baseline LDL-C, body mass index (BMI), HeFH status, and background therapies.
Clinical efficacy and safety in prevention of cardiovascular events
CLEAR Outcomes (Study 1002-043) was a multi-centre randomised, double-blind, placebocontrolled, event-driven trial in 13 970 adult patients with established atherosclerotic cardiovascular disease (CVD) (70%), or at high risk for atherosclerotic CVD (30%). Patients with established CVD had documented history of coronary artery disease, symptomatic peripheral arterial disease, and/or cerebrovascular atherosclerotic disease. Patients without established CVD were considered at high risk for CVD based on meeting at least one of the following criteria: (1) diabetes mellitus (type 1 or type 2) in women over 65 years of age, or men over 60 years of age, or (2) a Reynolds Risk score >30% or a SCORE Risk score >7.5% over 10 years, or 3) a coronary artery calcium score >400 Agatston units at any time in the past. Patients were randomised 1:1 to receive either Nilemdo 180 mg per day (n=6 992) or placebo (n=6 978) alone or as an add on to other background lipid lowering therapies that could include very low doses of statins. Overall, more than 95% of patients were followed until the end of the trial or death, and less than 1% were lost to follow up. The median follow-up duration was 3.4 years.
At baseline, the mean age was 65.5 years, 48% were women, 91% were White. Selected additional baseline characteristics included hypertension (85%), diabetes mellitus (46%), pre-diabetes mellitus (42%), current tobacco user (22%), eGFR <60 mL/min per 1.73 m² (21%), and a mean body mass index 29.9 kg/m². The mean baseline LDL-C was 3.6 mmol/L (139 mg/dL). At baseline, 41% of patients were taking at least one lipid modifying therapy including ezetimibe (12%), and very low dose of statins (23%).
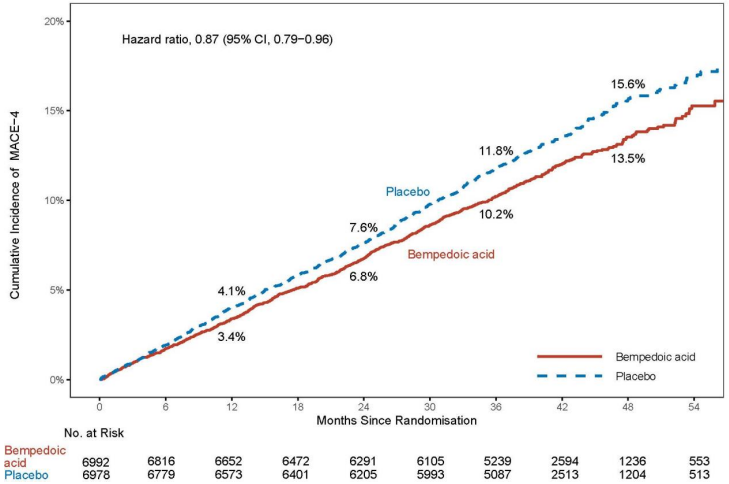
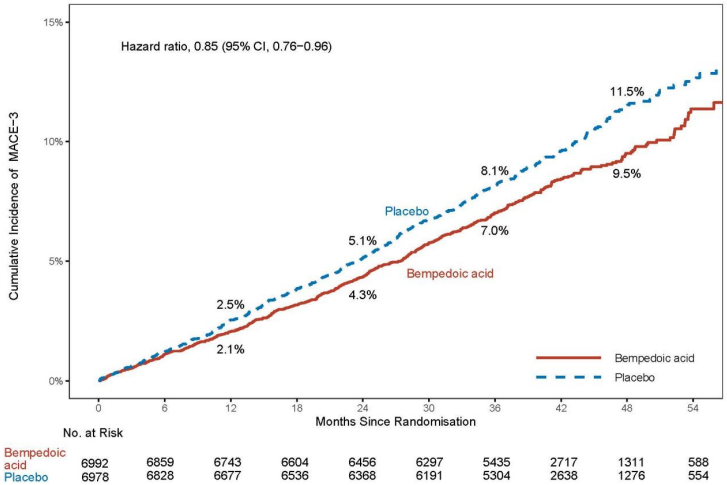
Nilemdo significantly reduced the risk for the primary composite endpoint of major adverse cardiovascular events (MACE-4) consisting of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, or coronary revascularization by 13% compared to placebo (Hazard Ratio: 0.87; 95% CI: 0.79, 0.96; p=0.0037); and the risk of the key secondary MACE-3 composite endpoint (cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) was significantly reduced by 15% compared to placebo (Hazard Ratio: 0.85; 95% CI: 0.76, 0.96; p=0.0058). The primary composite endpoint result was generally consistent across prespecified subgroups (including baseline age, race, ethnicity, sex, LDL-C category, statin use, ezetimibe use, and diabetes). Impact of Nilemdo on the individual components of the primary endpoint included a 27% reduction in the risk of nonfatal myocardial infarction and a 19% reduction in the risk of coronary revascularization compared to placebo. There was no statistically significant difference in the reduction of non-fatal stroke and risk of cardiovascular death compared to placebo. The results of the primary and key secondary efficacy endpoints are shown in Table 4. The Kaplan-Meier curve estimates of the cumulative incidence of the MACE-4 primary and the MACE-3 secondary endpoint are shown in Figures 1 and 2 below. The cumulative incidence of the MACE-4 primary endpoint is separated by month 6.
Further, the difference between Nilemdo and placebo in mean percent change in LDL-C from baseline to month 6 was -20% (95% CI: -21%, -19%).
Table 4. Effect of Nilemdo on Major Cardiovascular Events:
| Endpoint | Nilemdo N=6 992 | Placebo N=6 978 | Nilemdo vs. Placebo |
|---|---|---|---|
| n (%) | n (%) | Hazard Ratioa (95% CI) p-valueb | |
| Primary Composite Endpoint | |||
| Cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, coronary revascularization (MACE-4) | 819 (11.7) | 927 (13.3) | 0.87 (0.79, 0.96) 0.0037 |
| Components of Primary Endpoint | |||
| Non-fatal myocardial infarction | 236 (3.4) | 317 (4.5) | 0.73 (0.62, 0.87) |
| Coronary revascularization | 435 (6.2) | 529 (7.6) | 0.81 (0.72, 0.92) |
| Non-fatal stroke | 119 (1.7) | 144 (2.1) | 0.82 (0.64, 1.05) |
| Cardiovascular death | 269 (3.8) | 257 (3.7) | 1.04 (0.88, 1.24) |
| Key Secondary Endpoints | |||
| Cardiovascular death, non-fatal myocardial infarction, non-fatal stroke (MACE-3) | 575 (8.2) | 663 (9.5) | 0.85 (0.76, 0.96) 0.0058 |
| Fatal and non-fatal myocardial infarction | 261 (3.7) | 334 (4.8) | 0.77 (0.66, 0.91) 0.0016 |
| Coronary revascularization | 435 (6.2) | 529 (7.6) | 0.81 (0.72, 0.92) 0.0013 |
| Fatal and non-fatal stroke | 135 (1.9) | 158 (2.3) | 0.85 (0.67, 1.07) NS |
CI = confidence interval; MACE = major adverse cardiovascular event; NS=not significant
a Hazard ratio and corresponding 95% CI were based on a Cox proportional hazard model fitting treatment as explanatory variable.
b p-value was based on log rank test.
Note: this table also presents the time to first occurrence for each of the components of MACE; patients may be included in more than 1 category
Figure 1. Kaplan-Meier Curve for Time to First Occurrence of MACE-4:
MACE = major adverse cardiovascular event
Note: MACE-4 defined as the composite endpoint of CV death, non-fatal MI, non-fatal stroke, or coronary revascularization.
Figure 2. Kaplan-Meier Curve for Time to First Occurrence of MACE-3:
MACE = major adverse cardiovascular event
Note: MACE-3 defined as the composite endpoint of CV death, non-fatal MI, or non-fatal stroke.
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with bempedoic acid in paediatric population from 4 to less than 18 years of age in the treatment of elevated cholesterol. See section 4.2 for information on paediatric use.
5.2. Pharmacokinetic properties
Absorption
Pharmacokinetic data indicate that bempedoic acid is absorbed with a median time to maximum concentration of 3.5 hours when administered as Nilemdo 180 mg tablets. Bempedoic acid pharmacokinetic parameters are presented as the mean [standard deviation (SD)] unless otherwise specified. Bempedoic acid can be considered a prodrug that is activated intracellularly by ACSVL1 to ETC-1002-CoA. The steady-state Cmax and AUC following multiple dose administration in patients with hypercholesterolaemia were 24.8 (6.9) microgram/mL and 348 (120) microgram∙h/mL, respectively. Bempedoic acid steady-state pharmacokinetics were generally linear over a range of 120 mg to 220 mg. There were no time-dependent changes in bempedoic acid pharmacokinetics following repeat administration at the recommended dose, and bempedoic acid steady-state was achieved after 7 days. The mean accumulation ratio of bempedoic acid was approximately 2.3-fold.
Concomitant food administration had no effect on the oral bioavailability of bempedoic acid when administered as Nilemdo 180 mg tablets. Food slows the absorption rate of bempedoic acid; the absorption rate constant with food is 0.32/h.
Distribution
The bempedoic acid apparent volume of distribution (V/F) was 18 L. Plasma protein binding of bempedoic acid, its glucuronide and its active metabolite, ESP15228, were 99.3%, 98.8% and 99.2%, respectively. Bempedoic acid does not partition into red blood cells.
Biotransformation
In vitro metabolic interaction studies suggest that bempedoic acid, as well as its active metabolite and glucuronide forms are not metabolised by and do not inhibit or induce cytochrome P450 enzymes.
The primary route of elimination for bempedoic acid is through metabolism to the acyl glucuronide. Bempedoic acid is also reversibly converted to an active metabolite (ESP15228) based on aldo-keto reductase activity observed in vitro from human liver. Mean plasma AUC metabolite/parent drug ratio for ESP15228 following repeat-dose administration was 18% and remained constant over time. Both compounds are converted to inactive glucuronide conjugates in vitro by UGT2B7. Bempedoic acid, ESP15228 and their respective conjugated forms were detected in plasma with bempedoic acid accounting for the majority (46%) of the AUC0-48h and its glucuronide being the next most prevalent (30%). ESP15228 and its glucuronide represented 10% and 11% of the plasma AUC0-48h, respectively.
The steady-state Cmax and AUC of the equipotent active metabolite (ESP15228) of bempedoic acid in patients with hypercholesterolaemia were 3.0 (1.4) microgram/mL and 54.1 (26.4) microgram∙h/mL, respectively. ESP15228 likely made a minor contribution to the overall clinical activity of bempedoic acid based on systemic exposure and pharmacokinetic properties.
Elimination
The steady-state clearance (CL/F) of bempedoic acid determined from a population PK analysis in patients with hypercholesterolaemia was 12.1 mL/min after once-daily dosing; renal clearance of unchanged bempedoic acid represented less than 2% of total clearance. The mean (SD) half-life for bempedoic acid in humans was 19 (10) hours at steady-state.
Following single oral administration of 240 mg of bempedoic acid (1.3 times the approved recommended dose), 62.1% of the total dose (bempedoic acid and its metabolites) was recovered in urine, primarily as the acyl glucuronide conjugate of bempedoic acid, and 25.4% was recovered in faeces. Less than 5% of the administered dose was excreted as unchanged bempedoic acid in faeces and urine combined.
Special populations
Renal impairment
Pharmacokinetics of bempedoic acid was evaluated in a population PK analysis performed on pooled data from all clinical trials (n=2 261) to assess renal function on the steady-state AUC of bempedoic acid and in a single-dose pharmacokinetic study in subjects with varying degrees of renal function. Compared to patients with normal renal function, the mean bempedoic acid exposures were higher in patients with mild or moderate renal impairment by 1.4-fold (90% PI: 1.3, 1.4) and 1.9-fold (90% PI: 1.7, 2.0), respectively (see section 4.4).
There is limited information in patients with severe renal impairment; in a single dose study, the bempedoic acid AUC was increased by 2.4-fold in patients (n=5) with severe renal impairment (eGFR <30 mL/min/1.73 m²) compared to those with normal renal function. Clinical studies of bempedoic acid did not include patients with ESRD on dialysis (see section 4.4).
Hepatic impairment
The pharmacokinetics of bempedoic acid and its metabolite (ESP15228) was studied in patients with normal hepatic function or mild or moderate hepatic impairment (Child-Pugh A or B) following a single dose (n=8/group). Compared to patients with normal hepatic function, the bempedoic acid mean Cmax and AUC were decreased by 11% and 22%, respectively, in patients with mild hepatic impairment and by 14% and 16%, respectively, in patients with moderate hepatic impairment. This is not expected to result in lower efficacy. Therefore, no dose adjustment is necessary in patients with mild or moderate hepatic impairment.
Bempedoic acid was not studied in patients with severe hepatic impairment (Child-Pugh C).
Other special populations
The pharmacokinetics of bempedoic acid were not affected by age, gender, or race. Body weight was a statistically significant covariate. The lowest quartile of body weight (< 73 kg) was associated with an approximate 30% greater exposure. The increase in exposure was not clinically significant and no dose adjustments are recommended based on weight.
5.3. Preclinical safety data
The standard battery of genotoxicity studies has not identified any mutagenic or clastogenic potential of bempedoic acid. In full lifetime carcinogenicity studies in rodents, bempedoic acid increased the incidence of hepatocellular and thyroid gland follicular tumours in male rats and hepatocellular tumours in male mice. Because these are common tumours observed in rodent lifetime bioassays and the mechanism for tumourigenesis is secondary to a rodent-specific PPAR alpha activation, these tumours are not considered to translate to human risk.
Increased liver weight and hepatocellular hypertrophy were observed in rats only and were partially reversed after the 1-month recovery at ≥30 mg/kg/day or 4 times the exposure in humans at 180 mg. Reversible, non-adverse changes in laboratory parameters indicative of these hepatic effects, decreases in red blood cell and coagulation parameters, and increases in urea nitrogen and creatinine were observed in both species at tolerated doses. The NOAEL for adverse response in the chronic studies was 10 mg/kg/day and 60 mg/kg/day associated with exposures below and 15 times the human exposure at 180 mg in rats and monkeys, respectively.
Bempedoic acid was not teratogenic or toxic to embryos or foetuses in pregnant rabbits at doses up to 80 mg/kg/day or 12 times the systemic exposure in humans at 180 mg. Pregnant rats given bempedoic acid at 10, 30, and 60 mg/kg/day during organogenesis had decreased numbers of viable foetuses and reduced foetal body weight at ≥30 mg/kg/day or 4 times the systemic exposure in humans at 180 mg. An increased incidence of foetal skeletal findings (bent scapula and ribs) were observed at all doses, at exposures below the systemic exposure in humans at 180 mg. In a pre- and post-natal development study, pregnant rats administered bempedoic acid at 5, 10, 20 and 30 mg/kg/day throughout pregnancy and lactation had adverse maternal effects at ≥20 mg/kg/day and reductions in numbers of live pups and pup survival, pup growth and learning and memory at ≥10 mg/kg/day, with maternal exposures at 10 mg/kg/day, less than the exposure in humans at 180 mg.
No data are available on the effect of Nilemdo on human fertility. Administration of bempedoic acid to male and female rats prior to mating and through gestation day 7 in females resulted in changes in estrous cyclicity, decreased numbers of corpora lutea and implants at ≥30 mg/kg/day with no effects on male or female fertility or sperm parameters at 60 mg/kg/day (4 and 9 times the systemic exposure in humans at 180 mg, respectively).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.