NUCALA Powder for solution for injection Ref.[8924] Active ingredients: Mepolizumab
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: GlaxoSmithKline Trading Services Limited, 12 Riverwalk, Citywest Business Campus, Dublin 24, Ireland
Pharmacodynamic properties
Pharmacotherapeutic group: Drugs for obstructive airway diseases, other systemic drugs for obstructive airway diseases
ATC code: R03DX09
Mechanism of action
Mepolizumab is a humanised monoclonal antibody (IgG1, kappa), which targets human interleukin-5 (IL-5) with high affinity and specificity. IL-5 is the major cytokine responsible for the growth and differentiation, recruitment, activation and survival of eosinophils. Mepolizumab inhibits the bioactivity of IL-5 with nanomolar potency by blocking the binding of IL-5 to the alpha chain of the IL-5 receptor complex expressed on the eosinophil cell surface, thereby inhibiting IL-5 signalling and reducing the production and survival of eosinophils.
Pharmacodynamic effects
Severe eosinophilic asthma
In patients with severe refractory eosinophilic asthma (adults/adolescents), following a dose of 100 mg administered subcutaneously every 4 weeks for 32 weeks, blood eosinophils were reduced from a geometric mean count at baseline of 290 to 40 cells/μL at week 32 (n=182), a reduction of 84% compared to placebo.
This magnitude of blood eosinophils reduction was maintained in severe refractory eosinophilic asthma patients (n=998) treated for a median of 2.8 years (range 4 weeks to 4.5 years) in open-label extension studies.
In children aged 6 to 11 years old with severe refractory eosinophilic asthma administered mepolizumab subcutaneously every 4 weeks for 52 weeks, blood eosinophils were reduced from a geometric mean count at baseline to week 52 of 306 (n=16) to 48 (n=15) following 40 mg (for a weight <40kg) and 331 to 44 cells/μL (n=10) following 100 mg (for a weight ≥40 kg), a reduction from baseline of 85% and 87%, respectively.
In adults, adolescents and children, this magnitude of reduction was observed within 4 weeks of treatment.
CRSwNP
In patients with CRSwNP, following a 100 mg dose of mepolizumab administered subcutaneously every 4 weeks for 52 weeks, blood eosinophils were reduced from a geometric mean count at baseline to week 52 of 390 (n=206) to 60 cells/μL (n=126), which corresponds to a geometric mean reduction of 83% compared to placebo. This magnitude of reduction was observed within 4 weeks of treatment and was maintained throughout the treatment period of 52 weeks.
EGPA
In patients with EGPA, following a 300 mg dose of mepolizumab administered subcutaneously every 4 weeks for 52 weeks, blood eosinophils were reduced from a geometric mean count at baseline of 177 (n=68) to 38 cells/μL (n=64) at week 52. There was a geometric mean reduction of 83% compared to placebo and this magnitude of reduction was observed within 4 weeks of treatment.
HES
In patients with HES (adults/adolescents), following a 300 mg dose of mepolizumab administered subcutaneously every 4 weeks for 32 weeks, blood eosinophil reduction was observed within 2 weeks of treatment. At week 32, blood eosinophils were reduced from a geometric mean count at baseline of 1 460 (n=54) to 70 cells/μL (n=48) and a geometric mean reduction of 92% compared to placebo was observed. This magnitude of reduction was maintained for a further 20 weeks in patients that continued mepolizumab treatment in the open-label extension study.
Immunogenicity
Severe eosinophilic asthma, CRSwNP, EGPA and HES
Consistent with the potentially immunogenic properties of protein and peptide therapeutics, patients may develop antibodies to mepolizumab following treatment. In the placebo-controlled trials, 15/260 (6%) of adults and adolescents with severe refractory eosinophilic asthma treated with 100 mg dose, 6/196 (3%) of adults with CRSwNP treated with 100 mg dose, 1/68 (<2%) of adults with EGPA treated with 300 mg dose and 1/53 (2%) of adults and adolescents with HES treated with 300 mg dose of mepolizumab subcutaneously had detectable anti-mepolizumab antibodies after having received at least one dose of mepolizumab.
The immunogenicity profile of mepolizumab in severe refractory eosinophilic asthma patients (n=998) treated for a median of 2.8 years (range 4 weeks to 4.5 years) or in HES patients (n=102) treated for 20 weeks in open-label extension studies was similar to that observed in the placebo-controlled studies.
In children aged 6 to 11 years old with severe refractory eosinophilic asthma following either 40 mg subcutaneously (for a weight <40kg) or 100 mg subcutaneously (for a weight ≥40 kg), 2/35 (6%) had detectable anti-mepolizumab antibodies after having received at least one dose of mepolizumab during the initial short phase of the study. No children had detectable anti-mepolizumab antibodies during the long-term phase of the study. Neutralising antibodies were detected in one adult patient with severe refractory eosinophilic asthma and in no patients with CRSwNP, EGPA or HES. Anti-mepolizumab antibodies did not discernibly impact the pharmacokinetics and pharmacodynamics of mepolizumab in the majority of patients and there was no evidence of a correlation between antibody titres and change in blood eosinophil level.
Clinical efficacy
Severe eosinophilic asthma
The efficacy of mepolizumab in the treatment of a targeted group of patients with severe refractory eosinophilic asthma was evaluated in 3 randomised, double-blind, parallel-group clinical studies of between 24-52 weeks duration, in patients aged 12 years and older. These patients either remained uncontrolled (at least two severe exacerbations in the previous 12 months) on their current standard of care, including at least high doses of inhaled corticosteroids (ICS) plus an additional maintenance treatment(s), or were dependent on systemic corticosteroids. Additional maintenance treatments included long-acting beta2-adrenergic agonists (LABA), leukotriene modifiers, long-acting muscarinic antagonists (LAMA), theophylline, and oral corticosteroids (OCS).
The two exacerbations studies MEA112997 and MEA115588 enrolled a total of 1 192 patients, 60% females, with a mean age of 49 years (range 12–82). The proportion of patients on maintenance OCS was 31% and 24%, respectively. Patients were required to have a history of two or more severe asthma exacerbations requiring oral or systemic corticosteroid treatment in the past 12 months and reduced lung function at baseline (pre-bronchodilator FEV1<80% in adults and <90% in adolescents). The mean number of exacerbations in the previous year was 3.6 and the mean predicted pre-bronchodilator FEV1 was 60%. Patients continued to receive their existing asthma medicinal product during the studies.
For the oral corticosteroid-sparing study MEA115575, a total of 135 patients were enrolled (55% were female; mean age of 50 years) who were being treated daily with OCS (5-35 mg per day), and high-dose ICS plus an additional maintenance medicine.
Dose-ranging efficacy MEA112997 (DREAM) study
In MEA112997, a randomised, double-blind, placebo-controlled, parallel-group, multi-centre study of 52 weeks duration in 616 patients with severe refractory eosinophilic asthma, mepolizumab significantly reduced clinically significant asthma exacerbations (defined as worsening of asthma requiring use of oral/systemic corticosteroids and/or hospitalisation and/or emergency department visits) when administered in doses of 75 mg, 250 mg or 750 mg intravenously compared to placebo (see Table 1).
Table 1. Frequency of clinically significant exacerbations at week 52 in the intent to treat population:
| Intravenous mepolizumab | Placebo | |||
|---|---|---|---|---|
| 75 mg n=153 | 250 mg n=152 | 750 mg n=156 | n=155 | |
| Exacerbation rate/year | 1,24 | 1,46 | 1,15 | 2,40 |
| Percent reduction | 48% | 39% | 52% | |
| Rate ratio (95% CI) | 0,52 (0,39, 0,69) | 0,61(0,46, 0,81) | 0,48 (0,36, 0,64) | |
| p-value | <0,001 | <0,001 | <0,001 | - |
Exacerbation reduction MEA115588 (MENSA) study
MEA115588 was a randomised, double-blind, placebo-controlled, parallel-group, multi-centre study which evaluated the efficacy and safety of mepolizumab as add-on therapy in 576 patients with severe refractory eosinophilic asthma defined as peripheral blood eosinophils greater than or equal to 150 cells/μL at initiation of treatment or greater than or equal to 300 cells/μL within the past 12 months.
Patients received mepolizumab 100 mg administered subcutaneously, mepolizumab 75 mg administered intravenously or placebo treatment once every 4 weeks over 32 weeks. The primary endpoint was the frequency of clinically significant exacerbations of asthma and the reductions for both mepolizumab treatment arms compared to placebo were statistically significant (p<0.001). Table 2 provides the results of the primary and secondary endpoints for patients treated with subcutaneous mepolizumab or placebo.
Table 2. Results of primary and secondary endpoints at week 32 in the intent to treat population (MEA115588):
| Mepolizumab 100 mg (subcutaneous) N=194 | Placebo N=191 | |
|---|---|---|
| Primary endpoint | ||
| Frequency of clinically significant exacerbations | ||
| Exacerbation rate per year | 0.83 | 1.74 |
| Percent reduction Rate ratio (95% CI) | 53% 0.47 (0.35, 0.64) | - |
| p-value | <0.001 | |
| Secondary endpoints | ||
| Frequency of exacerbations requiring hospitalisations/emergency room visits | ||
| Exacerbation rate per year | 0.08 | 0.20 |
| Percent reduction Rate ratio (95% CI) | 61% 0.39 (0.18, 0.83) | - |
| p-value | 0.015 | |
| Frequency of exacerbations requiring hospitalisation | ||
| Exacerbations rate per year | 0.03 | 0.10 |
| Percent reduction Rate ratio (95% CI) | 69% 0.31 (0.11, 0.91) | - |
| p-value | 0.034 | |
| Pre-bronchodilator FEV1 (mL) at week 32 | ||
| Baseline (SD) | 1 730 (659) | 1 860 (631) |
| Mean Change from Baseline (SE) | 183 (31) | 86 (31) |
| Difference (mepolizumab vs. placebo) | 98 | |
| 95% CI | (11, 184) | |
| p-value | 0.028 | |
| St. George’s Respiratory Questionnaire (SGRQ) at week 32 | ||
| Baseline (SD) | 47.9 (19.5) | 46.9 (19.8) |
| Mean Change From Baseline (SE) | -16.0 (1.1) | -9.0 (1.2) |
| Difference (mepolizumab vs. placebo) | -7.0 | |
| 95% CI | (-10.2, -3.8) | |
| p-value | <0.001 | |
Reduction of exacerbation rate by baseline blood eosinophil count
Table 3 shows the results of a combined analysis of the two exacerbation studies (MEA112997 and MEA115588) by baseline blood eosinophil count. The rate of exacerbations in the placebo arm increased with increasing baseline blood eosinophil count. The reduction rate with mepolizumab was greater in patients with higher blood eosinophil counts.
Table 3. Combined analysis of the rate of clinically significant exacerbations by baseline blood eosinophil count in patients with severe refractory eosinophilic asthma:
| Mepolizumab 75 mg IV/100 mg SC N=538 | Placebo N=346 | |
|---|---|---|
| MEA112997+MEA115588 | ||
| <150 cells/μL | ||
| n | 123 | 66 |
| Exacerbation rate per year | 1.16 | 1.73 |
| Mepolizumab vs. placebo | ||
| Rate ratio (95% CI) | 0.67 (0.46, 0.98) | --- |
| 150 to <300 cells/μL | ||
| n | 139 | 86 |
| Exacerbation rate per year | 1.01 | 1.41 |
| Mepolizumab vs. placebo | ||
| Rate ratio (95% CI) | 0.72 (0.47, 1.10) | --- |
| 300 to <500 cells/μL | ||
| n | 109 | 76 |
| Exacerbation rate per year | 1.02 | 1.64 |
| Mepolizumab vs. placebo | ||
| Rate ratio (95% CI) | 0.62 (0.41, 0.93) | --- |
| ≥500 cells/μL | ||
| N | 162 | 116 |
| Exacerbation rate per year | 0.67 | 2.49 |
| Mepolizumab vs. placebo | ||
| Rate ratio (95% CI) | 0.27 (0.19, 0.37) | --- |
Oral corticosteroid reduction study MEA115575 (SIRIUS)
MEA115575 evaluated the effect of mepolizumab 100 mg administered subcutaneously on reducing the requirement for maintenance oral corticosteroids (OCS) while maintaining asthma control in subjects with severe refractory eosinophilic asthma. Patients had a blood eosinophil count of ≥150/μL at baseline or a blood eosinophil count of ≥300/μL in the 12 months prior to screening. Patients were administered mepolizumab or placebo treatment once every 4 weeks over the treatment period. Patients continued to receive their existing asthma medicinal product during the study with the exception of their OCS dose which was reduced every 4 weeks during the OCS reduction phase (Weeks 4-20), as long as asthma control was maintained.
A total of 135 patients were enrolled: mean age was 50 years, 55% were female, and 48% had been receiving oral steroid therapy for at least 5 years. The baseline mean prednisone equivalent dose was approximately 13 mg per day.
The primary endpoint was the percent reduction in daily OCS dose (weeks 20-24), whilst maintaining asthma control by defined dose reduction categories (see Table 4). Predefined categories included percent reductions ranging from 90-100% reduction, to no decrease in the prednisone dose from the end of the optimisation phase. The comparison between mepolizumab and placebo was statistically significant (p=0.008).
Table 4. Results of the primary and secondary endpoints in MEA115575:
| ITT Population | ||
|---|---|---|
| Mepolizumab 100 mg (subcutaneous) N=69 | Placebo N=66 | |
| Primary endpoint | ||
| Percent reduction in OCS from baseline (weeks 20-24) | ||
| 90% - 100% | 16 (23%) | 7 (11%) |
| 75% - <90% | 12 (17%) | 5 (8%) |
| 50% - <75% | 9 (13%) | 10 (15%) |
| >0% - <50% | 7 (10%) | 7 (11%) |
| No decrease in OCS/lack of asthma control/withdrawal from treatment | 25 (36%) | 37 (56%) |
| Odds ratio (95% CI) | 2,39 (1,25, 4,56) | |
| p-value | 0,008 | |
| Secondary endpoints (weeks 20-24) | ||
| Reduction in the daily OCS dose to 0 mg/d | 10 (14%) | 5 (8%) |
| Odds ratio (95% CI) | 1.67 (0.49, 5.75) | |
| p-value | 0. 414 | |
| Reduction in the daily OCS dose to ≤5mg/day | 37 (54%) | 21 (32%) |
| Odds ratio (95% CI) | 2.45 (1.12, 5.37) | |
| p-value | 0.025 | |
| Median % reduction in daily OCS dose from baseline (95% CI) | 50.0 (20.0, 75.0) | 0.0 (-20.0, 33.3) |
| Median difference (95% CI ) | -30.0 (-66.7, 0.0) | |
| p-value | 0.007 | |
Open-label extension studies in severe refractory eosinophilic asthma MEA115666 (COLUMBA), MEA115661 (COSMOS) and 201312 (COSMEX)
The long-term efficacy profile of mepolizumab in severe refractory eosinophilic asthma patients (n=998) treated for a median of 2.8 years (range 4 weeks to 4.5 years) in open-label extension studies MEA115666, MEA115661 and 201312 was generally consistent with the 3 placebo-controlled studies.
Chronic rhinosinusitis with nasal polyps (CRSwNP)
Study 205687 (SYNAPSE) was a 52-week, randomised, double-blind, placebo-controlled study which evaluated 407 patients aged 18 years and older with CRSwNP. Patients enrolled in the study were required to have a nasal obstruction VAS (Visual Analogue Scale) symptom score of >5 out of a maximum score of 10, an overall VAS symptom score >7 out of a maximum score of 10 and an endoscopic bilateral NP score of ≥5 out of a maximum score of 8 (with a minimum score of 2 in each nasal cavity). Patients must also have had a history of at least one prior surgery for nasal polyps in the previous 10 years.
Key baseline characteristics included total endoscopic NP score mean (SD) 5.5 (1.29), nasal obstruction VAS score mean (SD) 9.0 (0.83), overall VAS symptom score mean (SD) 9.1 (0.74), loss of smell VAS score mean (SD) 9.7 (0.72) and Sino-Nasal Outcome Test (SNOT-22) mean (SD) 64.1 (18.32). The geometric mean eosinophil count was 390 cells/mcL (95% CI: 360, 420). In addition, 27% of patients had aspirin-exacerbated respiratory disease (AERD) and 48% of patients had at least 1 course of OCS for CRSwNP in the past 12 months.
Patients received a 100 mg dose of mepolizumab or placebo, administered subcutaneously once every 4 weeks in addition to background intranasal corticosteroid therapy.
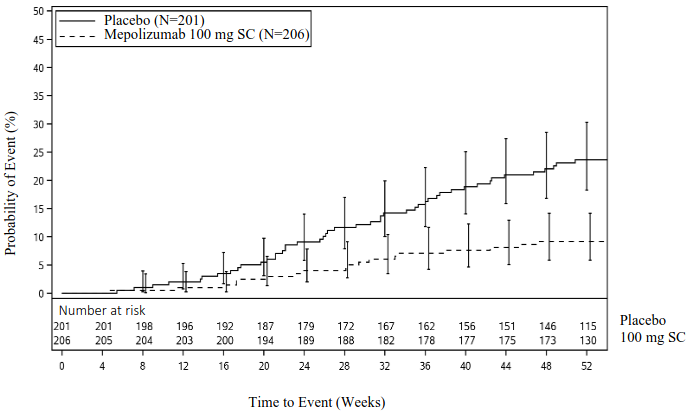
The co-primary endpoints were change from baseline in total endoscopic NP score at week 52 and change from baseline in mean nasal obstruction VAS score during weeks 49-52. The key secondary endpoint was the time to first NP surgery up to Week 52 (surgery was defined as any procedure involving instruments resulting in incision and removal of tissue [e.g. polypectomy] in the nasal cavity). Patients who received mepolizumab had significantly greater improvements (decreases) in total endoscopic NP score at Week 52 and in nasal obstruction VAS score during weeks 49-52 compared to placebo, and all secondary endpoints were statistically significant in favour of mepolizumab (see Table 5 and Figure 1).
Table 5. Summary of results for primary and secondary endpoints (intent to treat population):
| Placebo (N=201) | Mepolizumab 100 mg SC (N=206) | |
|---|---|---|
| Co-primary endpoints | ||
| Total endoscopic score at week 52a | ||
| Median score at baseline (min, max) | 6.0 (0, 8) | 5.0 (2, 8) |
| Median change from baseline | 0.0 | -1.0 |
| p-valueb | <0.001 | |
| Difference in medians (95% CI)c | -0.73 (-1.11, -0.34) | |
| ≥1-point improvement, n (%) | 57 (28) | 104 (50) |
| ≥2-point improvement, n (%) | 26 (13) | 74 (36) |
| Nasal obstruction VAS score (weeks 49 to 52)a | ||
| Median score at baseline (min, max) | 9.14 (5.31, 10.00) | 9.01 (6.54, 10.00) |
| Median change from baseline | -0.82 | -4.41 |
| p-valueb | <0.001 | |
| Difference in medians (95% CI)c | -3.14 (-4.09, -2.18) | |
| >1-point improvement, n (%) | 100 (50) | 146 (71) |
| ≥3-point improvement, n (%)d | 73 (36) | 124 (60) |
| Key secondary endpoint | ||
| Time to first nasal polyps surgery | ||
| Participants with surgery | 46 (23) | 18 (9) |
| Hazard ratio (Mepolizumab/Placebo) (95% CI)e | 0.43 (0.25, 0.76) | |
| p-valuee | 0.003 | |
| Other secondary endpoints | ||
| Overall VAS score (Weeks 49-52)a | ||
| Median score at baseline (min, max) | 9.20 (7.21, 10.00) | 9.12 (7.17, 10.00) |
| Median change from baseline | -0.90 | -4.48 |
| p-valueb | <0.001 | |
| Difference in medians (95% CI)c | -3.18 (-4.10, -2.26) | |
| ≥2.5-point improvement (%)f | 40 | 64 |
| SNOT-22 total score at week 52a,g | ||
| n | 198 | 205 |
| Median score at baseline (min, max) | 64.0 (19, 110) | 64.0 (17, 105) |
| Median change from baseline | -14.0 | -30.0 |
| p-valueb | <0.001 | |
| Difference in medians (95% CI)c | -16.49 (-23.57, -9.42) | |
| ≥28-point improvement (%)f | 32 | 54 |
| Patients requiring systemic corticosteroids for nasal polyps up to Week 52 | ||
| Number of patients with ≥1 course | 74 (37) | 52 (25) |
| Odds Ratio to Placebo (95% CI)h | 0.58 (0.36, 0.92) | |
| p-valueh | 0.020 | |
| Composite VAS score – nasal symptoms (Weeks 49-52)a,i | ||
| Median score at baseline (min, max) | 9.18 (6.03, 10.00) | 9.11 (4.91, 10.00) |
| Median change from baseline | -0.89 | -3.96 |
| p-valueb | <0.001 | |
| Difference in medians (95% CI)c | -2.68 (-3.44, -1.91) | |
| ≥2-point improvement (%)f | 40 | 66 |
| Loss of smell VAS score (Weeks 49-52)a | ||
| Median score at baseline (min, max) | 9.97 (6.69, 10.00) | 9.97 (0.94, 10.00) |
| Median change from baseline | 0.00 | -0.53 |
| p-valueb | <0.001 | |
| Difference in medians (95% CI)c | -0.37 (-0.65, -0.08) | |
| ≥3-point improvement (%)f | 19 | 36 |
a Patients with nasal surgery/sinuplasty prior to visit assigned their worst observed score prior to nasal surgery/sinuplasty. Those who withdrew from study with no nasal surgery/sinuplasty assigned their worst observed score prior to study withdrawal.
b Based on Wilcoxon rank-sum test.
c Quantile regression with covariates of treatment group, geographic region, baseline score and log(e) baseline blood eosinophil count.
d A three-point improvement in nasal obstruction VAS has been identified as a meaningful within-patient change for this assessment.
e Estimated from a Cox Proportional Hazards Model with covariates of treatment group, geographic region, baseline total endoscopic score (centrally read), baseline nasal obstruction VAS, log(e) baseline blood eosinophil count and number of previous surgeries (1, 2, >2 as ordinal).
f Threshold for improvement has been identified as a meaningful within-patient change for this assessment
g Improvement seen in all 6 domains of symptoms and impact associated with CRSwNP.
h Analysis using logistic regression model with covariates of treatment group, geographic region, number of OCS courses for NP in last 12 months (0, 1, >1 as ordinal), baseline total Endoscopic Nasal Polyps score (centrally read), baseline nasal obstruction VAS score and log(e) baseline blood eosinophil count.
i Composite VAS score of nasal obstruction, nasal discharge, mucus in the throat and loss of smell.
Time to first NP surgery
Across the 52-week treatment period, patients in the mepolizumab group had a lower probability of undergoing NP surgery than patients in the placebo group. The risk of surgery over the treatment period was significantly lower by 57% for patients treated with mepolizumab compared with placebo (Hazard Ratio: 0.43; 95% CI 0.25, 0.76; p=0.003).
Figure 1. Kaplan Meier Curve for Time to First Nasal Polyps Surgery:
A post-hoc analysis of the proportion of patients with surgery showed a 61% reduction in the odds of surgery versus placebo (OR: 0.39, 95% CI: 0.21, 0.72; p=0.003).
CRSwNP patients with co-morbid asthma
In 289 (71%) patients with co-morbid asthma, pre-specified analyses showed improvements in the co-primary endpoints consistent with those seen in the overall population in the patients who received mepolizumab 100 mg compared with placebo. Additionally in these patients, there was a greater improvement from baseline at Week 52 in asthma control as measured by the Asthma Control Questionnaire (ACQ-5) for mepolizumab 100 mg compared with placebo (median change [Q1, Q3] of -0.80 [-2.20, 0.00] and 0.00 [-1.10, 0.20], respectively).
Eosinophilic granulomatosis with polyangiitis (EGPA)
MEA115921 was a randomised, double-blind, placebo-controlled, 52-week study which evaluated 136 adult patients with EGPA, who had a history of relapsing or refractory disease, and who were on stable oral corticosteroid therapy (OCS; ≥7.5 to ≤50 mg/day prednisolone/prednisone), with or without stable immunosuppressant therapy (excluding cyclophosphamide). Other background standard of care therapy was allowed during the study. Fifty-three percent (n=72) were also on concomitant stable immunosuppressant therapy. Patients with organ threatening or life-threatening EGPA were excluded from study MEA115921.
Patients either received a 300 mg dose of mepolizumab or placebo administered subcutaneously once every 4 weeks in addition to their background prednisolone/prednisone with or without immunosuppressive therapy. The OCS dose was tapered at the discretion of the investigator.
Remission
The co-primary endpoints were the total accrued duration of remission, defined as a Birmingham Vasculitis Activity Score (BVAS) =0 plus prednisolone/prednisone dose ≤4 mg/day, and the proportion of patients in remission at both 36 and 48 weeks of treatment. BVAS=0 represents no active vasculitis.
Compared with placebo, patients receiving mepolizumab 300 mg achieved a significantly greater accrued time in remission. Additionally, compared to placebo, a significantly higher proportion of patients receiving mepolizumab 300 mg achieved remission at both Week 36 and Week 48 (Table 6).
For both co-primary endpoints, compared with placebo, the beneficial effect observed following mepolizumab 300 mg treatment was present irrespective of if patients were receiving immunosuppressant therapy in addition to background corticosteroids.
Using the secondary endpoint remission definition of BVAS=0 plus prednisolone/prednisone ≤7.5 mg/day, patients receiving mepolizumab 300 mg also achieved significantly greater accrued time in remission (p<0.001), and a higher proportion of patients were in remission at both Week 36 and Week 48 (p<0.001), compared to placebo.
Table 6. Analyses of Co-Primary Endpoints:
| Number (%) of patients | ||
|---|---|---|
| Placebo N=68 | Mepolizumab 300mg N=68 | |
| Accrued Duration of Remission Over 52 Weeks | ||
| 0 | 55 (81) | 32 (47) |
| >0 to <12 weeks | 8 (12) | 8 (12) |
| 12 to <24 weeks | 3 (4) | 9 (13) |
| 24 to <36 weeks | 0 | 10 (15) |
| ≥36 weeks | 2 (3) | 9 (13) |
| Odds ratio (mepolizumab/placebo) | 5.91 | |
| 95% CI | --- | 2.68, 13.03 |
| p-value | --- | <0.001 |
| Patients in remission at Weeks 36 and 48 | 2 (3) | 22 (32) |
| Odds ratio (mepolizumab/placebo) | 16.74 | |
| 95% CI | --- | 3.61, 77.56 |
| p-value | --- | <0.001 |
An odds ratio >1 favours Nucala. Remission: BVAS=0 and OCS dose ≤4mg/day.
Relapse
Compared with placebo, the time to first relapse was significantly longer for patients receiving mepolizumab 300 mg (p<0.001). Additionally, patients receiving mepolizumab had a 50% reduction in annualised relapse rate compared with placebo: 1.14 vs 2.27, respectively.
Oral corticosteroid reduction
Patients treated with mepolizumab had a significantly lower average daily OCS during Weeks 48-52 compared with patients who received placebo. During Weeks 48 to 52, 59% and 44% of patients treated with mepolizumab achieved an average daily OCS dose of ≤7.5 mg and ≤4 mg respectively compared with 33% and 7% in the placebo group. 18% of patients in the mepolizumab group were able to taper off OCS completely compared with 3% in the placebo group.
Asthma Control Questionnaire–6 (ACQ-6)
Patients treated with mepolizumab had significant improvements in mean ACQ 6 score during Weeks 49-52 compared with patients who received placebo.
Hypereosinophilic syndrome (HES)
Study 200622 was a randomised, double-blind, placebo-controlled, 32-week study which evaluated 108 patients ≥12 years old with HES. Patients received 300 mg of mepolizumab, or placebo administered subcutaneously once every 4 weeks while continuing their HES therapy. In study 200622, HES therapy included but was not limited to OCS, immunosuppressive, cytotoxic therapy or other symptomatic therapies associated with HES such as omeprazole.
Patients entering the study had experienced at least two HES flares within the past 12 months and had a blood eosinophil count ≥1000 cells/μL during screening. Patients who were FIP1L1-PDGFRα kinase-positive were excluded from the study.
The primary endpoint of study 200622 was the proportion of patients who experienced a HES flare during the 32-week treatment period. A HES flare was defined as worsening of clinical signs and symptoms of HES resulting in the need to increase OCS or increase/add cytotoxic or immunosuppressive HES therapy or receiving blinded active OCS due to increased blood eosinophils (on ≥2 occasions).
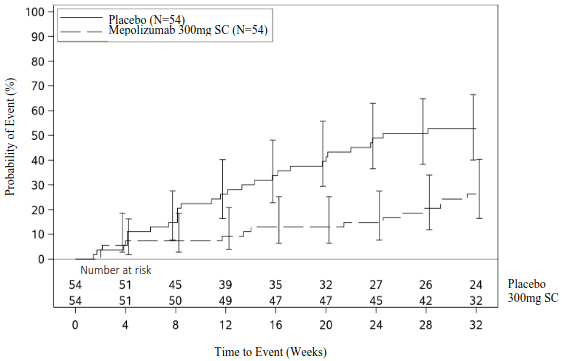
The primary analysis compared patients who experienced a HES flare or withdrew from the study in the mepolizumab and placebo treatment groups. Over the 32-week treatment period, 50% fewer patients experienced a HES flare or withdrew from the study when treated with 300 mg mepolizumab compared with placebo; 28% versus 56% respectively (OR 0.28, 95% CI: 0.12, 0.64) (see Table 7). Secondary endpoints were time to first HES flare, proportion of patients who experienced a HES flare during Week 20 through Week 32, rate of HES flares and change from baseline in fatigue severity.
All secondary endpoints were statistically significant and provided support for the primary endpoint (see Figure 2 and Table 8).
Table 7. Results of primary endpoint/analysis in the Intent to Treat population (Study 200622):
| Mepolizumab 300 mg N=54 | Placebo N=54 | |
|---|---|---|
| Proportion of patients who experienced a HES flare | ||
| Patients with ≥1 HES flare or who withdrew from study (%) | 15 (28) | 30 (56) |
| Patients with ≥1 HES flare (%) | 14 (26) | 28 (52) |
| Patients with no HES flare who withdrew (%) | 1 (2) | 2 (4) |
| Odds ratio (95% CI) | 0.28 (0.12, 0.64) | |
| CMH p-value | 0.002 | |
CMH = Cochran-Mantel-Haenszel
Figure 2. Kaplan Meier Curve for Time to First HES Flare:
Table 8. Results of other secondary endpoints in the Intent to Treat population (Study 200622):
| Mepolizumab 300 mg N=54 | Placebo N=54 | |
|---|---|---|
| HES flares during week 20 and up to and including week 32 | ||
| Patients with ≥1 HES flare or who withdrew from study (%) | 9 (17) | 19 (35) |
| Odds ratio (95% CI) | 0.33 (0.13, 0.85) | |
| CMH p-value | 0.02 | |
| Rate of HES flares | ||
| Estimated mean rate/year | 0.50 | 1.46 |
| Rate ratio (95% CI)a | 0.34 (0.19, 0.63) | |
| Wilcoxon Rank Sum Test p-value | 0.002 | |
| Change from baseline in fatigue severity based on Brief Fatigue Inventory (BFI) Item 3 (worst level of fatigue during past 24 hours) at week 32b | ||
| Median change in BFI item 3 | -0.66 | 0.32 |
| Comparison (mepolizumab vs. placebo) Wilcoxon Rank Sum Test p-value | 0.036 | |
a rate ratio <1 favours mepolizumab.
b patients with missing data included with worst observed value. BFI item 3 scale: 0 = no fatigue to 10 = as bad as you can imagine
CMH = Cochran-Mantel-Haenszel
Open-label extension (OLE)
Study 205203 was a 20-week open-label extension of Study 200622. HES therapy was allowed to be adjusted per local standard of care while maintaining mepolizumab 300 mg treatment starting at Week 4. In this study the effect of treatment with mepolizumab on the reduction of HES flares reported during Study 200622 was sustained for patients who continued mepolizumab treatment in study 205203, in which 94% (47/50) of patients did not experience a flare. In the 72 patients requiring OCS during Weeks 0 to 4 of the OLE, 28% of patients achieved a mean daily dose OCS dose reduction of ≥50% during Weeks 16 to 20.
Paediatric population
Severe refractory eosinophilic asthma
In MEA115588 and in the double-blind placebo-controlled study 200862, there were 34 adolescents (12 to 17 years old). Of these 34 subjects: 12 received placebo, 9 received mepolizumab 75 mg intravenously, and 13 received 100 mg subcutaneously. In a combined analysis of these studies, a 40% reduction in clinically significant exacerbations was observed in adolescents following mepolizumab treatment compared to placebo (rate ratio 0.60; 95% CI: 0.17, 2.10).
Eosinophilic granulomatosis with polyangiitis (EGPA)
The are no clinical data available in children and adolescents aged 6 to 17 years old.
HES
Four adolescents (12 to 17 years old) were enrolled in study 200622; one adolescent received mepolizumab 300 mg, and 3 adolescents received placebo for 32 weeks. The one adolescent treated with mepolizumab in the 32-week Study 200622 did not have a HES flare. All 4 adolescents that completed study 200622 continued into a 20-week open-label extension study 205203 in which one of the 4 adolescents experienced one HES flare.
Pharmacokinetic properties
Following subcutaneous dosing in patients with asthma and CRSwNP, mepolizumab exhibited approximately dose-proportional pharmacokinetics over a dose range of 12.5 mg to 250 mg. Subcutaneous administration of mepolizumab 300 mg had approximately three times the systemic exposure of mepolizumab 100 mg.
Absorption
Following subcutaneous administration to healthy subjects or patients with asthma, mepolizumab was absorbed slowly with a median time to reach maximum plasma concentration (Tmax) ranging from 4 to 8 days.
Following a single subcutaneous administration in the abdomen, thigh or arm of healthy subjects, mepolizumab absolute bioavailability was 64%, 71% and 75%, respectively. In patients with asthma the absolute bioavailability of mepolizumab administered subcutaneously in the arm ranged from 74-80%. Following repeat subcutaneous administration every 4 weeks, there is approximately a two-fold accumulation at steady state.
Distribution
Following a single intravenous administration to patients with asthma, mepolizumab distributes into a mean volume of distribution of 55 to 85 mL/kg.
Biotransformation
Mepolizumab is a humanised IgG1 monoclonal antibody degraded by proteolytic enzymes which are widely distributed in the body and not restricted to hepatic tissue.
Elimination
Following a single intravenous administration to patients with asthma, the mean systemic clearance (CL) ranged from 1.9 to 3.3 mL/day/kg, with a mean terminal half-life of approximately 20 days. Following subcutaneous administration of mepolizumab the mean terminal half-life (t1/2) ranged from 16 to 22 days. In the population pharmacokinetic analysis estimated mepolizumab systemic clearance was 3.1 mL/day/kg.
Special populations
Elderly patients (≥65 years old)
There are limited pharmacokinetic data available in elderly patients (≥65 years old) across all clinical studies (N=90). However, in the population pharmacokinetic analysis, there were no indications of an effect of age on the pharmacokinetics of mepolizumab over the age range of 12 to 82 years.
Renal impairment
No formal studies have been conducted to investigate the effect of renal impairment on the pharmacokinetics of mepolizumab. Based on population pharmacokinetic analyses, no dose adjustment is required in patients with creatinine clearance values between 50-80 mL/min. There are limited data available in patients with creatinine clearance values <50 mL/min.
Hepatic impairment
No formal studies have been conducted to investigate the effect of hepatic impairment on the pharmacokinetics of mepolizumab. Since mepolizumab is degraded by widely distributed proteolytic enzymes, not restricted to hepatic tissue, changes in hepatic function are unlikely to have any effect on the elimination of mepolizumab.
Paediatric population
Severe eosinophilic asthma and HES:
There are limited pharmacokinetic data available in the paediatric population (59 patients with eosinophilic esophagitis, 55 patients with severe refractory eosinophilic asthma and 1 patient with HES). Intravenous mepolizumab pharmacokinetics was evaluated by population pharmacokinetic analysis in a paediatric study conducted in patients aged 2–17 years old with eosinophilic esophagitis. Paediatric pharmacokinetics was largely predictable from adults, after taking into account bodyweight. Mepolizumab pharmacokinetics in adolescent patients with severe refractory eosinophilic asthma or HES included in the phase 3 studies were consistent with adults (see section 4.2).
Paediatric pharmacokinetics following subcutaneous administration in patients 6 to 11 years old with severe refractory eosinophilic asthma was investigated in an open label, uncontrolled study of 12-weeks duration. Paediatric pharmacokinetics were broadly consistent with adults and adolescents after accounting for bodyweight and bioavailability. The absolute subcutaneous bioavailability appears complete compared to that observed in adults and adolescents of 76%. Exposure following subcutaneous administration of either 40 mg (for a weight <40kg) or 100 mg (for a weight ≥40 kg) was 1.32 and 1.97 times of that observed in adults at 100 mg.
Investigation of a 40 mg subcutaneous dosing regimen administered every 4 weeks in children 6 to 11 years old over a 15-70 kg broad weight range by PK modelling and simulation predicts that the exposure of this dosing regimen would remain on average within 38% of adults at 100 mg. This dosing regimen is considered acceptable due to the wide therapeutic index of mepolizumab.
EGPA:
Mepolizumab pharmacokinetics in children (6 to 17 years old) with EGPA were predicted using modelling and simulation, based on pharmacokinetics in other eosinophilic diseases, and are expected to be consistent with those observed in children with severe eosinophilic asthma. The recommended posology in children 6 to 11 years old over a 15-70 kg broad weight range predicts that the exposure would remain on average within 26% of adults at 300 mg.
Preclinical safety data
As mepolizumab is a monoclonal antibody, no genotoxicity or carcinogenicity studies have been conducted.
Animal toxicology and/or pharmacology
Non-clinical data reveal no special hazards for humans based on conventional studies of safety pharmacology or repeated dose toxicity studies in monkeys. Intravenous and subcutaneous administration to monkeys was associated with reductions in peripheral and lung eosinophil counts, with no toxicological findings.
Eosinophils are thought to be associated with immune system responses to some parasitic infections. Studies conducted in mice treated with anti-IL-5 antibodies or genetically deficient in IL-5 or eosinophils have not shown impaired ability to clear parasitic infections. The relevance of these findings for humans is unknown.
Fertility
No impairment of fertility was observed in a fertility and general reproduction toxicity study in mice performed with an analogous antibody that inhibits IL-5 in mice. This study did not include a littering or functional offspring assessment.
Pregnancy
In monkeys, mepolizumab had no effect on pregnancy or on embryonic/fetal and postnatal development (including immune function) of the offspring. Examinations for internal or skeletal malformations were not performed. Data in cynomolgus monkeys demonstrate that mepolizumab crossed the placenta. Concentrations of mepolizumab were about 1.2-2.4 times higher in infants than in mothers for several months post partum and did not affect the immune system of the infants.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.