OBIZUR Powder and solvent for solution for injection Ref.[7646] Active ingredients: Susoctocog alfa
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: Baxalta Innovations GmbH, Industriestrasse 67, A-1221, Vienna, Austria
Therapeutic indications
Treatment of bleeding episodes in patients with acquired haemophilia caused by antibodies to Factor VIII.
OBIZUR is indicated in adults.
Posology and method of administration
Treatment with OBIZUR should be under the supervision of a physician experienced in the treatment of haemophilia.
The product is for in-patient administration only. It requires clinical supervision of the bleeding status of the patient.
Posology
The dose, frequency, and duration of the therapy with OBIZUR depend on the location, extent and severity of the bleeding episode, target Factor VIII activity, and on the patient’s clinical condition.
The number of units of Factor VIII administered is expressed in Units (U) that are derived from an in-house standard that has been calibrated with the current WHO standard for Factor VIII products. One Unit (U) of Factor VIII activity is equivalent to that quantity of Factor VIII in one ml of normal human plasma.
The recommended initial dose is 200 U per kilogram bodyweight, given by intravenous injection (see section 6.6). The required initial dose of OBIZUR for a patient is calculated using the following formula:
Initial dose (U/kg) / Product strength (U/vial) × Body weight (kg) = Number of vials
e.g. for a 70 kg subject the number of vials for an initial dose will be calculated as follows:
200 U/kg / 500 U/vial × 70 kg = 28 vials
Monitor Factor VIII activity and clinical condition 30 minutes after the first injection and 3 hours after administering OBIZUR.
Monitor Factor VIII activity immediately prior to and 30 minutes after subsequent doses and refer to
the table below for recommended target Factor VIII trough levels. The one-stage clotting assay for Factor VIII is recommended as it has been used in determination of the potency of OBIZUR and the mean recovery rate (see section 4.4 and 5.2).
The dose and frequency of administration should be based on results of Factor VIII activity (to be maintained within recommended limits) and on the clinical response achieved.
Efficacy and safety data in patients with acquired haemophilia are limited (see section 5.1).
Initial Phase
| Type of Bleeding | Target Factor VIII Trough Activity (Units per dL or % of normal) | Initial Dose (Units per kg) | Subsequent Dose | Frequency and Duration of Subsequent Dosing |
|---|---|---|---|---|
| Mild and moderate superficial muscle / no neurovascular compromise and joint bleeding | >50% | 200 | Titrate subsequent doses based on clinical response and to maintain target Factor VIII trough activity | Dose every 4 to 12 hours, frequency may be adjusted based on clinical response and measured Factor VIII activity |
| Major moderate to severe intramuscular, retroperitoneal, gastrointestinal, intracranial bleeding | >80% |
Healing phase
Once bleeding has responded, usually within the first 24 hours, continue OBIZUR with a dose that maintains the trough FVIII activity at 30-40% until bleeding is controlled. The maximum blood FVIII activity must not exceed 200%.
The length of treatment depends on clinical judgement.
Paediatric population
Use in children and adolescents below 18 years with congenital or in rare cases of acquired haemophilia is currently not approved.
Method of administration
Intravenous use.
The total volume of reconstituted OBIZUR should be administered at a rate of 1 to 2 mL per minute.
For instructions on reconstitution of the medicinal product before administration, see section 6.6.
Overdose
The effects of higher than recommended doses of OBIZUR have not been characterised.
Shelf life
Shelf life: 30 months.
The reconstituted solution should be used immediately, but no longer than 3 hours after reconstitution.
Special precautions for storage
Store in a refrigerator (2°C–8°C). Do not freeze.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
Nature and contents of container
One pack of OBIZUR contains 1, 5 or 10 each of the following:
- powder vials (type I glass) with a stopper (butyl rubber) and a flip-off seal
- pre-filled (type I glass) syringes with a stopper (butyl rubber), a rubber tip cap and a Luer Lock adapter
- fluid transfer device with an integral plastic spike
Special precautions for disposal and other handling
After reconstitution, the solution is clear, colourless, free from particles and has a pH of 6.8 to 7.2. The osmolality of the formulation buffer ranges between 59 and 65 10% mOsm/kg H2O.
Reconstituted medicinal product should be inspected visually for particulate matter and discoloration prior to administration. Solutions with particles or discolouration must not be administered.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Preparation
Before starting reconstitution you will need the following:
- Calculated number of powder vials
- Same number of 1 mL solvent syringes and sterile vial adapters
- Alcohol swabs
- Large sterile syringe to contain the final volume of reconstituted product
The procedures below are provided as general guidelines for the preparation and reconstitution of OBIZUR. Repeat following reconstitution instructions for each powder vial to be reconstituted.
Reconstitution
- Use aseptic technique during the reconstitution procedure.
- Bring the OBIZUR powdervial and the pre-filled solvent syringe to room temperature.
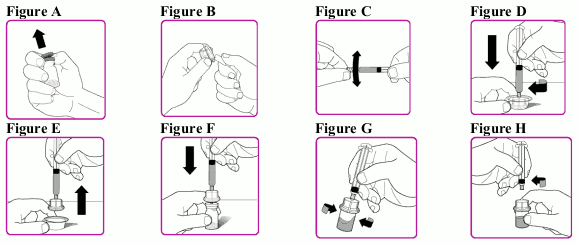
- Remove the plastic cap from the OBIZUR powder vial (Figure A).
- Wipe the rubber stopper with an alcohol swab (not supplied) and allow it to dry prior to use.
- Peel back the cover of the vial adapter package (Figure B). Do not to touch the luer lock (tip) in the centre of the vial adapter. Do not remove the vial adapter from the package.
- Place the vial adapter package on a clean surface with the luer lock pointing up.
- Snap off the tamper resistant cap of the pre-filled solvent syringe (Figure C).
- While firmly holding the vial adapter package connect the pre-filled solvent syringe to the vial adapter by pushing the syringe tip down onto the luer lock in the centre of the vial adapter, and turning it clockwise until the syringe is secured. Do not over tighten (Figure D).
- Remove the plastic package (Figure E).
- Place the OBIZUR powder vial on a clean, flat, hard surface. Place the vial adapter over the OBIZUR powder vial and firmly push the filter spike of the vial adapter through the centre of the OBIZUR powder vial’s rubber circle until the clear plastic cap snaps onto the vial (Figure F).
- Push the plunger down to slowly inject all of the diluent from the syringe into the OBIZUR powder vial.
- Gently swirl (in a circular motion) the OBIZUR powder vial without removing the syringe until all of the powder is fully dissolved/reconstituted (Figure G). The reconstituted solution should be inspected visually for particulate matter before administration. Do not use if particulate matter or discoloration is observed.
- With one hand hold the vial and vial adapter, and with the other hand firmly grasp the barrel of the pre-filled solvent syringe and in a counterclockwise motion unscrew the syringe from the vial adapter (Figure H).
- Use OBIZUR immediately and within 3 hours after reconstitution when stored at room temperature.
Administration
For intravenous injection only!
- Inspect the reconstituted OBIZUR solution for particulate matter and discoloration prior to administration. The solution should be clear and colorless in appearance. Do not administer if particulate matter or discoloration is observed.
- Do not administer OBIZUR in the same tubing or container with other medicinal products for injection.
Using aseptic technique, administer using the following procedure:
1. Once all vials have been reconstituted, connect a large syringe to the vial adapter by gently pushing the syringe tip down onto the luer lock in the centre of the vial adapter, and turning clockwise until the syringe is secured.
2. Invert the vial; push the air in the syringe into the vial and withdraw the reconstituted OBIZUR into the syringe (Figure I).
3. Unscrew the large syringe counterclockwise from the vial adapter, and repeat this process for all reconstituted vials of OBIZUR until the total volume to be administered is reached.
4. Administer the reconstituted OBIZUR intravenously at a rate of 1 to 2 mL per minute.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.