OJJAARA Tablet Ref.[107232] Active ingredients: Momelotinib
Source: FDA, National Drug Code (US) Revision Year: 2023
12.1. Mechanism of Action
Momelotinib is an inhibitor of wild type Janus Kinase 1 and 2 (JAK1/JAK2) and mutant JAK2V617F, which contribute to signaling of a number of cytokines and growth factors that are important for hematopoiesis and immune function. Momelotinib and its major human circulating metabolite, M21, have higher inhibitory activity for JAK2 compared to JAK3 and tyrosine kinase 2 (TYK2). Momelotinib and M21 additionally inhibit activin A receptor type 1 (ACVR1), also known as activin receptor like kinase 2 (ALK2), which produces subsequent inhibition of liver hepcidin expression and increased iron availability resulting in increased red blood cell production. MF is a myeloproliferative neoplasm associated with constitutive activation and dysregulated JAK signaling that contributes to inflammation and hyperactivation of ACVR1. JAK signaling recruits and activates STAT (signal transducers and activation of transcription) proteins resulting in nuclear localization and subsequent regulation of gene transcription.
12.2. Pharmacodynamics
Momelotinib inhibited STAT3 phosphorylation in whole blood from patients with MF. Maximal inhibition of STAT3 phosphorylation occurred 2 hours after momelotinib dosing and inhibition persisted for at least 6 hours [see Clinical Pharmacology (12.1)]. Iron availability and erythropoiesis was assessed by analysis of circulating hepcidin concentrations. An acute and sustained reduction of circulating hepcidin was observed for the duration of the 24-week administration of momelotinib to patients with MF [see Clinical Pharmacology (12.1)].
Cardiac Electrophysiology
Momelotinib did not prolong the QT interval to any clinically relevant extent at 4 times the highest recommended dosage of 200 mg.
12.3. Pharmacokinetics
Momelotinib pharmacokinetic parameters are presented as mean (%CV) and were derived in patients with MF unless otherwise specified.
The momelotinib steady-state Cmax is 479 ng/mL (61%) and AUC is 3,288 ng•h/mL (60%) at the maximum recommended dosage. Momelotinib exposure (i.e., Cmax and AUC) increases dose proportionally from 100 mg to 300 mg (0.5 to 1.5 times the maximum recommended dosage), but less than dose‑proportional at doses from 400 mg to 800 mg (2 to 4 times the maximum recommended dosage). There is no clinically significant accumulation.
Absorption
Median time to momelotinib Cmax (Tmax) at steady state is 2 hours (Q1: 1 hour; Q3: 3 hours) post dose.
Effect of Food
No clinically significant differences in momelotinib pharmacokinetics were observed following administration of either a high-fat meal (800 kcal; 50% fat) or low-fat meal (400 kcal; 20% fat) in healthy subjects.
Distribution
Momelotinib steady state apparent volume of distribution is 984 L (118%). Momelotinib plasma protein binding is approximately 91% in healthy volunteers.
Elimination
The elimination half-life of momelotinib and the M21 metabolite is 4 to 8 hours. Momelotinib clearance is 103 L/h (87%).
Metabolism
Momelotinib is metabolized by multiple cytochrome P450 (CYP) enzymes including CYP3A4 (36%), CYP2C8 (19%), CYP2C9 (17%), CYP2C19 (19%), and CYP1A2 (9%).
M21 is an active human metabolite that has approximately 40% of the pharmacological activity of the parent. M21 is formed by CYP followed by aldehyde oxidase metabolism of momelotinib. The mean M21 to momelotinib ratio for AUC ranged from 1.4 to 2.1.
Excretion
Following a single oral dose of radiolabeled momelotinib, 69% (13% unchanged) of radioactivity was excreted in feces and 28% (<1% unchanged) in urine. Approximately 12% of the administered dose was excreted in urine as M21.
Specific Populations
No clinically significant differences in momelotinib and M21 pharmacokinetics were observed based on age (range: 28 to 92 years), race (83% White, 6% Asian, 2% Black), sex (60% male), weight (range: 34 kg to 138 kg), renal impairment (eGFR: 16.4 mL/min/1.73 m² to above 120 mL/min/1.73 m²), or mild or moderate hepatic impairment (Child-Pugh A or B). The effect of end stage renal disease receiving dialysis on momelotinib pharmacokinetics is unknown.
Patients with Hepatic Impairment
Momelotinib Cmax increased by 13% and AUC increased by 97% in subjects with severe hepatic impairment (Child-Pugh C). The M21 metabolite Cmax decreased by 76% and AUC decreased by 48% in subjects with severe hepatic impairment (Child-Pugh C).
Drug Interaction Studies
Clinical Studies
OATP1B1/1B3 Inhibitors:
Momelotinib Cmax increased by 40% and AUC increased by 57% following concomitant use with a single dose of a OATP1B1/1B3 inhibitor (rifampin). The M21 metabolite Cmax increased by 6% and AUC increased by 12%.
BCRP Substrates:
A BCRP substrate (rosuvastatin) Cmax increased by 220% and AUC increased by 170% following concomitant use of a single dose of rosuvastatin at 10 mg with multiple doses of momelotinib (200 mg once daily).
Other Drugs:
No clinically significant differences in momelotinib and M21metabolite pharmacokinetics were observed when used concomitantly with a strong CYP3A4 inducer (tested with multiple-dose rifampin), a strong CYP3A4 inhibitor (ritonavir), or an acid reducing agent (omeprazole, a proton pump inhibitor).
No clinically significant differences in the pharmacokinetics of a CYP3A4 substrate (midazolam) were observed when used concomitantly with momelotinib.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenicity potential of momelotinib was assessed in rasH2 transgenic mice and Sprague-Dawley rats. There was no evidence of tumorigenicity in male or female mice that received momelotinib doses up to 100 mg/kg/day for 26 weeks. In a 2-year oral carcinogenicity study in Sprague-Dawley rats, momelotinib caused benign Leydig cell tumors at a dose of 15 mg/kg/day (17 times the maximum recommended dose based on combined momelotinib and M21 AUC). The increase in Leydig cell adenomas was considered related to a rat-specific phenomenon (i.e., prolactin-dependent Leydig cell tumorigenesis).
Momelotinib was not mutagenic in a bacterial reverse mutation assay, or clastogenic in an in vitro chromosomal aberration assay with human peripheral blood lymphocytes or in vivo in a rat bone marrow micronucleus assay.
In fertility studies in rats, momelotinib was administered for at least 70 days (males) and 14 days (females) prior to cohabitation and up to the implantation day (gestation Day 7) at doses of 5, 25, and 68 mg/kg/day. Momelotinib reduced sperm concentration and motility and reduced testes and seminal vesicle weights at 25 mg/kg/day or greater (approximately 13 times the recommended dose based on combined momelotinib and M21 AUC) leading to reduced fertility at 68 mg/kg/day. In females, momelotinib reduced ovarian function (reproductive cycles and ovulation) at 68 mg/kg/day and decreased the number of pregnant females and increased pre- and post-implantation loss with most pregnant rats having total litter loss at 25 mg/kg/day or greater. Exposures at the NOAEL in male and female rats at 5 mg/kg/day are approximately 3-times the recommended dose (based on combined momelotinib and M21 AUC).
14. Clinical Studies
The efficacy of OJJAARA in the treatment of adults with intermediate 1, intermediate 2, or high-risk MF, including primary MF, post-PV MF or post-ET MF, as defined by the Dynamic International Prognostic Scoring System (DIPSS) or International Prognostic Scoring System (IPSS) for MF, was established in the MOMENTUM trial and in a subpopulation of adults with anemia in the SIMPLIFY-1 trial. All patients received a starting dosage of OJJAARA 200 mg once daily. Eligible patients had baseline platelet count of ≥25 × 109/L in MOMENTUM and ≥50 × 109/L in SIMPLIFY-1.
MOMENTUM
MOMENTUM (NCT04173494) was a double-blind, 2:1 randomized, active-controlled trial in 195 symptomatic and anemic adults with MF who had previously received an approved JAK inhibitor therapy. Patients were treated with OJJAARA 200 mg once daily or danazol 300 mg twice daily for 24 weeks, then switched to open-label treatment with OJJAARA.
The median age was 71 years (range 38 to 86 years) with 79% of patients aged 65 years and older, and 63% of patients were male. Overall, 81% of patients were White, 9% of patients were Asian, 2% of patients were Black, and 6% of patients were Hispanic or Latino. Sixty-four percent of patients had primary MF, 19% had post-PV MF, and 17% had post-ET MF. Five percent of patients had intermediate-1 risk, 57% had intermediate-2 risk, and 35% had high-risk disease. Within the 8 weeks prior to treatment, 79% of patients had received red blood cell (RBC) transfusions (median of 4 RBC units; interquartile range: 1-6). At baseline, 13% and 15% of patients were transfusion independent (defined as no red blood cell transfusions in the 12 weeks before the first dose and Hb ≥8 g/dL) in the OJJAARA and danazol groups, respectively. The baseline median Hb count was 8 g/dL and the median platelet count was 96 × 109/L (range 24 × 109/L to 733 × 109/L). The baseline median palpable spleen length was 11 cm below the left costal margin; the median central spleen volume measured by magnetic resonance imaging (MRI) or computed tomography (CT) was 2,105 cm3 (range 609 cm3 to 9,717 cm3).
Symptoms were measured using the Myelofibrosis Symptom Assessment Form (MFSAF v4.0) diary. The MFSAF v4.0 patient diary, completed throughout the randomized treatment period, captured the core symptoms of MF: fatigue (weariness and tiredness), night sweats (or feeling hot or flushed), itching, abdominal discomfort (feeling pressure or bloating), pain under ribs on left side, feeling of fullness after beginning to eat, and bone pain. For each item, symptom scores, ranging from 0 (absent) to 10 (worst imaginable), were added to create a daily Total Symptom Score (maximum score of 70). At baseline, the mean MFSAF v4.0 Total Symptom Score was 28 in the OJJAARA group and 26 in the danazol group.
The efficacy of OJJAARA in the treatment of patients with primary or secondary MF and anemia was established based on a significantly higher percentage of patients treated with OJJAARA compared to danazol achieving a MFSAF v4.0 Total Symptom Score reduction of 50% or more at Week 24 compared with their own baseline score (Table 4). Other endpoints included transfusion independence, spleen volume response, MFSAF v4.0 Total Symptom Score change from baseline, and percentage of patients with no transfusions.
Table 4. Percent of Patients Achieving Symptom Reduction, Transfusion Independence, and Spleen Volume Reduction at Week 24 in MOMENTUM:
| OJJAARA n=130 | Danazol n=65 | p-value | |
|---|---|---|---|
| Patients with MFSAF v4.0 Total Symptom Score Reduction of 50% or more, n (%) | 32 (25%) | 6 (9%) | <0.01 |
| Treatment differencea (95% CI) | 16% (6, 26) | ||
| Patients with Transfusion Independence (no transfusion or Hb <8 g/dL between Weeks 12 and 24), n (%) | 39 (30%) | 13 (20%) | 0.023 |
| Non-inferiority treatment differencea,b (95% CI) | 14% (2, 25) | ||
| Patients with Spleen Volume Reduction by 25% or More, n (%) | 51 (39%) | 4 (6%) | <0.0001 |
| Treatment differencea (95% CI) | 33% (23, 44) | ||
| MFSAF v4.0 Total Symptom Score Change from Baselineb (SE) | -9.4 (1.1) | -3.1 (1.6) | 0.001 |
| Treatment differencec (95% CI) | -6.2 (-10, -2.4) | ||
| Patients with Spleen Volume Reduction by 35% or More, n (%) | 29 (22%) | 2 (3%) | 0.001 |
| Treatment differencea (95% CI) | 18% (10, 27) | ||
| Patients with No Transfusionsc,d (during the 24-week treatment period), n (%) | 46 (35%) | 11 (17%) | 0.001 |
| Treatment differencea (95% CI) | 17% (8, 26) | ||
a Analyses stratified by baseline MFSAF v4.0 Total Symptom Score (<22 vs. ≥22), baseline palpable spleen length below the left costal margin (<12 vs. ≥12 cm), and baseline red blood cell or whole blood units transfused in the 8-week period before randomization (0, 1-4, ≥5 units).
b Non-inferiority difference between OJJAARA response rate and 80% of danazol response rate.
c Least squares means and difference are reported.
d Eight subjects treated with OJJAARA and 3 subjects treated with danazol had no transfusion, but discontinued treatment prior to Week 24.
Figure 1 shows the percentage of patients treated with OJJAARA and danazol who achieved a 50% or greater reduction from baseline for each individual symptom in the MFSAF v4.0.
Figure 1. Percent of Patients Achieving a 50% or Greater Reduction in Individual MFSAF v4.0 Symptom Scores at Week 24a in MOMENTUM:
QD = once daily; BID = twice a day.
a Thirty-six (27.7%) subjects treated with OJJAARA and 27 (41.5%) subjects treated with danazol discontinued treatment prior to Week 24.
SIMPLIFY-1
SIMPLIFY-1 (NCT01969838) was a double-blind, randomized, active-controlled trial in 432 adults with MF who had not previously received a JAK inhibitor. Patients were treated with OJJAARA 200 mg once daily or ruxolitinib adjusted dose twice daily for 24 weeks. Patients were eligible to switch to open-label OJJAARA after 24 weeks (without tapering of the JAK inhibitor received during the randomization period). The baseline characteristics and efficacy results provided are for the subset of patients who had anemia (Hb <10 g/dL) at baseline (n=181).
The median age was 68 years (range 25 to 86 years) with 67% of patients aged 65 years and older, and 59% of patients were male. Eighty-one percent of patients were White, 8% of patients were Asian, 1% of patients were Black, and 2% of patients were Hispanic or Latino. Sixty-three percent of patients had primary MF, 13% had post-PV MF, and 24% had post-ET MF. Four percent of patients had intermediate-1 risk, 25% had intermediate-2 risk, and 71% had high-risk disease. At baseline, 29% and 44% of patients were transfusion independent in the groups treated with OJJAARA or ruxolitinib, respectively. The baseline median Hb measurement was 8.8 g/dL and the median platelet count was 193 × 109/L (range 54 × 109/L to 2,865 × 109/L). Median palpable spleen length at baseline was 12 cm below the left costal margin; the median spleen volume at baseline (measured by MRI or CT) was 1,843 cm3 (range 352 cm3 to 9,022 cm3).
The efficacy of OJJAARA in the treatment of patients with MF in SIMPLIFY-1 was based on spleen volume response (reduction by 35% or greater). A numerically lower percent of patients treated with OJJAARA (25%) achieved a Total Symptom Score reduction of 50% or more at Week 24 compared with ruxolitinib (36%).
The spleen volume reduction results are presented in Table 5.
Table 5. Percent of Patients a Achieving 35% or Greater Reduction from Baseline in Spleen Volume at Week 24 in SIMPLIFY-1:
| OJJAARA n=86 | Ruxolitinib n=95 | |
|---|---|---|
| Patients with Spleen Volume Reduction by 35% or More, n (%) (95% CI) | 27 (31.4%) (21.8, 42.3) | 31 (32.6%) (23.4, 43.0) |
a Subset of patients with anemia (Hb <10 g/dL) at baseline. |
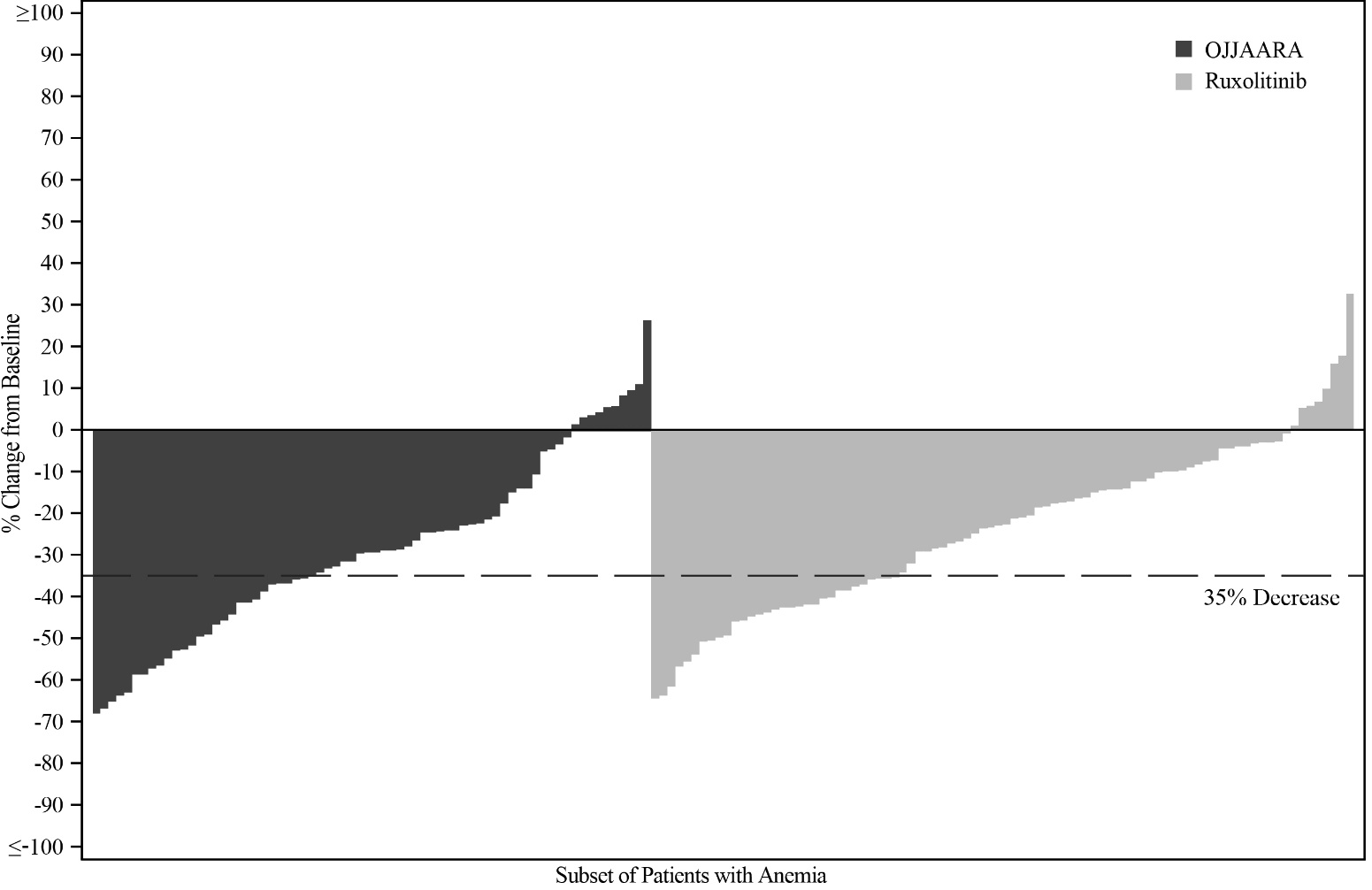
Figure 2 shows the percent change from baseline in spleen volume for each patient at Week 24 in SIMPLIFY-1.
Figure 2. Percent Change from Baseline in Spleen Volume for Each Patient at Week 24 in SIMPLIFY-1a,b:
a Subset of patients with anemia (Hb <10 g/dL) at baseline.
b Missing data rates for OJJAARA and ruxolitinib were 19% and 8%.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.