OLATUTON Powder and solvent for suspension for injection Ref.[49976] Active ingredients: Octreotide
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2022 Publisher: TEVA UK Limited, Brampton Road, Hampden Park, Eastbourne, BN22 9AG, United Kingdom
4.1. Therapeutic indications
Treatment of patients with acromegaly in whom surgery is inappropriate or ineffective, or in the interim period until radiotherapy becomes fully effective (see section 4.2).
Treatment of patients with symptoms associated with functional gastro-entero-pancreatic endocrine tumours e.g. carcinoid tumours with features of the carcinoid syndrome (see section 5.1).
Treatment of patients with advanced neuroendocrine tumours of the midgut or of unknown primary origin where non-midgut sites of origin have been excluded.
Treatment of TSH-secreting pituitary adenomas:
- when secretion has not normalised after surgery and/or radiotherapy;
- in patients in whom surgery is inappropriate;
- in irradiated patients, until radiotherapy is effective.
4.2. Posology and method of administration
Posology
Acromegaly
It is recommended to start treatment with the administration of 20 mg Olatuton at 4-week intervals for 3 months. Patients on treatment with s.c. octreotide can start treatment with Olatuton the day after the last dose of s.c. octreotide. Subsequent dosage adjustment should be based on serum growth hormone (GH) and insulin-like growth factor 1/somatomedin C (IGF-1) concentrations and clinical symptoms.
For patients in whom, within this 3-month period, clinical symptoms and biochemical parameters (GH; IGF-1) are not fully controlled (GH concentrations still above 2.5 microgram/L), the dose may be increased to 30 mg every 4 weeks. If after 3 months, GH, IGF-1, and/or symptoms are not adequately controlled at a dose of 30 mg, the dose may be increased to 40 mg every 4 weeks.
For patients whose GH concentrations are consistently below 1 microgram/L, whose IGF-1 serum concentrations normalised, and in whom most reversible signs/symptoms of acromegaly have disappeared after 3 months of treatment with 20 mg, 10 mg Olatuton may be administered every 4 weeks. However, particularly in this group of patients, it is recommended to closely monitor adequate control of serum GH and IGF-1 concentrations, and clinical signs/symptoms at this low dose of Olatuton.
For patients on a stable dose of Olatuton, assessment of GH and IGF-1 should be made every 6 months.
Gastro-entero-pancreatic endocrine tumours
Treatment of patients with symptoms associated with functional gastro-entero-pancreatic neuroendocrine tumours
It is recommended to start treatment with the administration of 20 mg Olatuton at 4-week intervals. Patients on treatment with s.c. octreotide should continue at the previously effective dosage for 2 weeks after the first injection of Olatuton.
For patients in whom symptoms and biological markers are well controlled after 3 months of treatment, the dose may be reduced to 10 mg Olatuton every 4 weeks.
For patients in whom symptoms are only partially controlled after 3 months of treatment, the dose may be increased to 30 mg Olatuton every 4 weeks.
For days when symptoms associated with gastro-entero-pancreatic tumours may increase during treatment with Olatuton, additional administration of s.c. octreotide is recommended at the dose used prior to the Olatuton treatment. This may occur mainly in the first 2 months of treatment until therapeutic concentrations of octreotide are reached.
Treatment of patients with advanced neuroendocrine tumours of the midgut or of unknown primary origin where non-midgut sites of origin have been excluded
The recommended dose of Olatuton is 30 mg administered every 4 weeks (see section 5.1). Treatment with Olatuton for tumour control should be continued in the absence of tumour progression.
Treatment of TSH-secreting adenomas
Treatment with Olatuton should be started at a dose of 20 mg at 4-weekly intervals for 3 months before considering dose adjustment. The dose is then adjusted on the basis of the TSH and thyroid hormone response.
Use in patients with impaired renal function
Impaired renal function did not affect the total exposure (AUC) to octreotide when administered s.c. Therefore, no dose adjustment of Olatuton is necessary.
Use in patients with impaired hepatic function
In a study with octreotide administered s.c. and i.v. it was shown that the elimination capacity may be reduced in patients with liver cirrhosis, but not in patients with fatty liver disease. In certain cases patients with impaired hepatic function may require dose adjustment.
Use in the elderly
In a study with octreotide administered s.c., no dose adjustment was necessary in subjects ≥65 years of age. Therefore, no dose adjustment is necessary in this group of patients with Olatuton.
Use in children
There is limited experience with the use of Olatuton in children.
Method of administration
Olatuton may only be administered by deep intramuscular injection. The site of repeat intramuscular injections should be alternated between the left and right gluteal muscle (see section 6.6).
4.9. Overdose
A limited number of accidental overdoses of octreotide prolonged-release injection have been reported. The doses ranged from 100 mg to 163 mg/month of octreotide prolonged-release injection. The only adverse event reported was hot flushes.
Cancer patients receiving doses of octreotide prolonged-release injection up to 60 mg/month and up to 90 mg/2 weeks have been reported. These doses were in general well tolerated; however, the following adverse events have been reported: frequent urination, fatigue, depression, anxiety, and lack of concentration.
The management of overdosage is symptomatic.
6.3. Shelf life
3 years.
The product must not be stored after reconstitution (must be used immediately).
6.4. Special precautions for storage
Store in the original package in order to protect from light.
Store in a refrigerator (2°C-8°C). Do not freeze.
Olatuton may be stored below 25°C on the day of injection.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
6.5. Nature and contents of container
Each unit contains one glass vial with rubber stopper (chlorobutyl rubber), sealed with an aluminium cap with a dark blue flip-off seal, containing powder for suspension for injection and one colourless pre-filled glass syringe with tip cap and plunger stopper (bromobutyl rubber) with 2 ml of solvent, co-packaged in a plastic tray with one vial adapter and one safety injection needle.
Packs of one and three units are available.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Instructions for preparation and intramuscular injection for Olatuton
FOR DEEP INTRAMUSCULAR INJECTION ONLY
Included in the injection kit:
a. One vial containing Olatuton powder
b. One prefilled syringe containing the vehicle solution for reconstitution
c. One vial adapter for drug product reconstitution
d. One safety injection needle.
Follow the instructions below carefully to ensure proper reconstitution of Olatuton before deep intramuscular injection.
There are 3 critical actions in the reconstitution of Olatuton. Not following them could result in failure to deliver the drug appropriately.
- The injection kit must reach room temperature. Remove the injection kit from the fridge and let the kit stand at room temperature for a minimum of 30 minutes before reconstitution, but do not exceed 24 hours.
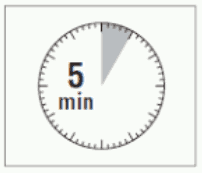
- After adding the diluent solution, ensure that the powder is fully saturated by letting the vial stand for 5 minutes.
- After saturation, shake the vial moderately in a horizontal direction for a minimum of 30 seconds until a uniform suspension is formed. The Olatuton suspension must only be prepared immediately before administration.
Olatuton should only be administered by a trained healthcare professional.
Step 1:
- Remove the Olatuton injection kit from refrigerated storage.
ATTENTION: It is essential to start the reconstitution process only after the injection kit reaches room temperature. Let the kit stand at room temperature for a minimum of 30 minutes before reconstitution, but do not exceed 24 hours.
Note: The injection kit can be re-refrigerated if needed.
Step 2:
- Remove the plastic cap from the vial and clean the rubber stopper of the vial with an alcohol wipe.
- Peel the blister film and remove the vial adapter from its packaging by holding between the white luer cap and the skirt.
DO NOT touch the tip of the access device at any place.
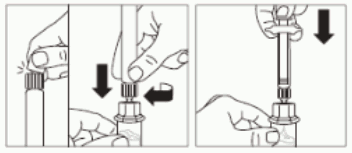
- Place the vial on a flat surface.
Position the vial adapter on top of the vial and push it fully down so that it snaps in place, confirmed by an audible “click”.
- Clean the tip of the vial adapter with an alcohol wipe.
Step 3:
- Snap off the smooth white cap from the syringe prefilled with diluent solution and screw the syringe onto the vial adapter.
- Slowly push the plunger all the way down to transfer all the diluent solution in the vial.
Step 4:
ATTENTION: It is essential to let the vial stand for 5 minutes to ensure that the diluent has fully saturated the powder.
Note: It is normal if the plunger rod moves up as there might be a slight overpressure in the vial.
- At this stage prepare the patient for injection.
Step 5:
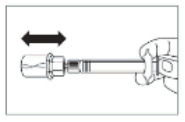
- After the saturation period, make sure that the plunger is pushed all the way down in the syringe.
ATTENTION: Keep the plunger pressed and shake the vial moderately in a horizontal direction for a minimum of 30 seconds so that the powder is completely suspended (uniform milky suspension). Repeat moderate shaking for another 30 seconds if the powder is not completely suspended.
Step 6:
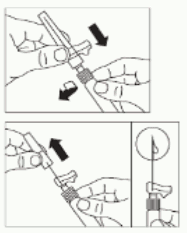
- Turn syringe and vial upside down, slowly pull the plunger back and draw the entire contents from the vial into the syringe.
- Unscrew the syringe from the vial adapter.
Step 7:
- Prepare the injection site with an alcohol wipe.
- Screw the safety injection needle onto the syringe.
- If immediate administration is delayed, gently re-shake the syringe to ensure a milky uniform suspension.
- Pull the protective cover straight off the needle.
- Gently tap the syringe to remove any visible bubbles and expel them from the syringe.
- Proceed immediately to Step 8 for administration to the patient.
Any delay may result in sedimentation.
Step 8:
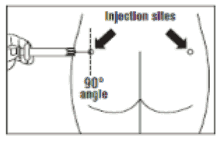
- Olatuton must be given only by deep intramuscular injection, NEVER intravenously.
- Insert the needle fully into the left or right gluteus at a 90° angle to the skin.
- Slowly pull back the plunger to check that no blood vessel has been penetrated (reposition if a blood vessel has been penetrated).
- Depress the plunger with steady pressure until the syringe is empty.
Withdraw the needle from the injection site and activate the safety guard (as shown in Step 9).
Step 9:
- Activate the safety guard over the needle in one of the 2 methods shown:
- either press the hinged section of the safety guard down onto a hard surface (figure A)
- or push the hinge forward with your finger (figure B).
- An audible “click” confirms the proper activation.
- Note: Record injection site on patient’s record and alternate monthly
- Dispose of syringe immediately (in a sharps container).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.