OLUMIANT Film-coated tablet Ref.[6377] Active ingredients: Baricitinib
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Eli Lilly Nederland B.V., Papendorpseweg 83, 3528BJ Utrecht, The Netherlands
Pharmacodynamic properties
Pharmacotherapeutic group: Immunosuppressants, selective immunosuppressants
ATC code: L04AF02
Mechanism of action
Baricitinib is a selective and reversible inhibitor of Janus kinase (JAK)1 and JAK2. In isolated enzyme assays, baricitinib inhibited the activities of JAK1, JAK2, Tyrosine Kinase 2 and JAK3 with IC50 values of 5.9, 5.7, 53 and >400 nM, respectively.
Janus kinases (JAKs) are enzymes that transduce intracellular signals from cell surface receptors for a number of cytokines and growth factors involved in haematopoiesis, inflammation and immune function. Within the intracellular signalling pathway, JAKs phosphorylate and activate signal transducers and activators of transcription (STATs), which activate gene expression within the cell. Baricitinib modulates these signalling pathways by partially inhibiting JAK1 and JAK2 enzymatic activity, thereby reducing the phosphorylation and activation of STATs.
Pharmacodynamic effects
Inhibition of IL-6 induced STAT3 phosphorylation
Administration of baricitinib resulted in a dose dependent inhibition of IL-6 induced STAT3 phosphorylation in whole blood from healthy subjects with maximal inhibition observed 2 hours after dosing which returned to near baseline by 24 hours.
Immunoglobulins
Mean serum IgG, IgM, and IgA values decreased by 12 weeks after starting treatment, and remained stable at a lower value than baseline through at least 104 weeks. For most patients, changes in immunoglobulins occurred within the normal reference range.
Lymphocytes
Mean absolute lymphocyte count increased by 1 week after starting treatment, returned to baseline by week 24, and then remained stable through at least 104 weeks. For most patients, changes in lymphocyte count occurred within the normal reference range.
C-reactive protein
In patients with rheumatoid arthritis, decreases in serum C-reactive protein (CRP) were observed as early as 1 week after starting treatment and were maintained throughout dosing.
Creatinine
In clinical trials, baricitinib induced a mean increase in serum creatinine levels of 3.8 μmol/L after two weeks of treatment, which remained stable thereafter. This may be due to inhibition of creatinine secretion by baricitinib in the renal tubules. Consequently, estimates of the glomerular filtration rate based on serum creatinine may be slightly reduced, without actual loss of renal function or the occurrence of renal adverse reactions. In alopecia areata, mean serum creatinine continued to increase up to week 52. In atopic dermatitis and alopecia areata, baricitinib was associated with a decrease in cystatin C (also used to estimate glomerular filtration rate) at week 4, with no further decreases thereafter.
In vitro skin models
In an in vitro human skin model treated with pro-inflammatory cytokines (i.e., IL-4, IL-13, IL-31), baricitinib reduced epidermal keratinocyte pSTAT3 expression, and increased the expression of filaggrin, a protein that plays a role in skin barrier function and in the pathogenesis of atopic dermatitis.
Vaccine study
The influence of baricitinib on the humoral response to non-live vaccines was evaluated in 106 rheumatoid arthritis patients under stable treatment with baricitinib 2 or 4 mg, receiving inactivated pneumococcal or tetanus vaccination. The majority of these patients (n=94) were co-treated with methotrexate. For the total population, pneumococcal vaccination resulted in a satisfactory IgG immune response in 68% (95% CI: 58.4%, 76.2%) of the patients. In 43.1% (95% CI: 34%, 52.8%) of the patients, a satisfactory IgG immune response to tetanus vaccination was achieved.
Clinical efficacy
Rheumatoid arthritis
The efficacy and safety of baricitinib once daily were assessed in 4 Phase III randomised, double-blind, multicentre studies in adult patients with moderate to severe active rheumatoid arthritis diagnosed according to the ACR/EULAR 2010 criteria (Table 3). The presence of at least 6 tender and 6 swollen joints was required at baseline. All patients who completed these studies were eligible to enrol in a long term extension study for up to 7 years additional treatment.
Table 3. Clinical trial summary:
| Study name (Duration) | Population (Number) | Treatment arms | Summary of key outcome measures |
|---|---|---|---|
| RA-BEGIN (52 weeks) | MTX-naïve1 (584) | • Baricitinib 4 mg QD • Baricitinib 4 mg QD + MTX • MTX | • Primary endpoint: ACR20 at week 24 • Physical function (HAQ-DI) • Radiographic progression (mTSS) • Low disease activity and Remission (SDAI) |
| RA-BEAM (52 weeks) | MTX-IR2 (1305) | • Baricitinib 4 mg QD • Adalimumab 40 mg SC Q2W • Placebo All patients on background MTX | • Primary endpoint:ACR20 at week 12 • Physical function (HAQ-DI) • Radiographic progression (mTSS) • Low disease activity and Remission (SDAI) • Morning Joint Stiffness |
| RA-BUILD (24 weeks) | cDMARD-IR3 (684) | • Baricitinib 4 mg QD • Baricitinib 2 mg QD • Placebo On background cDMARDs5 if on stable cDMARD at study entry | • Primary endpoint: ACR20 at week 12 • Physical function (HAQ-DI) • Low disease activity and remission (SDAI) • Radiographic progression (mTSS) • Morning Joint Stiffness |
| RA-BEACON (24 weeks) | TNF-IR4 (527) | • Baricitinib 4 mg QD • Baricitinib 2 mg QD • Placebo On background cDMARDs5 | • Primary endpoint: ACR20 at week 12 • Physical function (HAQ-DI) • Low disease activity and Remission (SDAI) |
Abbreviations: IR = inadequate responder; QD = Once daily; Q2W = Once every 2 weeks; SC = Subcutaneously; ACR = American College of Rheumatology; SDAI = Simplified Disease Activity Index; HAQ-DI = Health Assessment Questionnaire-Disability Index; mTSS = modified Total Sharp Score
1 Patients who had received less than 3 doses of Methotrexate (MTX); naïve to other conventional or biologic DMARDs
2 Patients who had an inadequate response to MTX (+/- other cDMARDs); biologic-naïve
3 Patients who had an inadequate response or were intolerant to ≥1 cDMARDs; biologic-naïve
4 Patients who had an inadequate response or were intolerant to ≥1 bDMARDs; including at least one TNF inhibitor
5 Most common concomitant cDMARDs included MTX, hydroxychloroquine, leflunomide and sulfasalazine
Clinical response
In all studies, patients treated with baricitinib 4 mg once daily had statistically significantly higher ACR20, ACR50 and ACR70 response at 12 weeks compared to placebo, methotrexate (MTX) or adalimumab (Table 4). Time to onset of efficacy was rapid across measures with significantly greater responses seen as early as week 1. Continued, durable response rates were observed, with ACR20/50/70 responses maintained for at least 2 years including the long-term extension study.
Treatment with baricitinib 4 mg, alone or in combination with cDMARDs, resulted in significant improvements in all individual ACR components, including tender and swollen joint counts, patient 17 and physician global assessments, HAQ-DI, pain assessment and CRP, compared to placebo, MTX or adalimumab.
No relevant differences regarding efficacy and safety were observed in subgroups defined by types of concomitant DMARDs used in combination with baricitinib.
Remission and low disease activity
A statistically significantly greater proportion of patients treated with baricitinib 4 mg compared to placebo or MTX achieved remission (SDAI ≤3.3 and CDAI ≤2.8) or low disease activity or remission (DAS28-ESR or DAS28-hsCRP ≤3.2 and DAS28-ESR or DAS28-hsCRP <2.6), at weeks 12 and 24 (Table 4).
Greater rates of remission compared to placebo were observed as early as week 4. Remission and low disease activity rates were maintained for at least 2 years. Data from the long-term extension study up to 6 years follow-up indicate durable low disease activity/remission rates.
Table 4. Response, remission and physical function:
| Study | RA-BEGIN MTX-naïve patients | RA-BEAM MTX-IR patients | RA-BUILD cDMARD-IR patients | RA-BEACON TNF-IR patients | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment group | MTX | BARI 4 mg | BARI 4 mg + MTX | PBO | BARI 4 mg | ADA 40 mg Q2W | PBO | BARI 2 mg | BARI 4 mg | PBO | BARI 2 mg | BARI 4 mg |
| N | 210 | 159 | 215 | 488 | 487 | 330 | 228 | 229 | 227 | 176 | 174 | 177 |
| ACR20 | ||||||||||||
| Week 12 | 59% | 79%*** | 77%*** | 40% | 70%†*** | 61%*** | 39% | 66%*** | 62%*** | 27% | 49%*** | 55%*** |
| Week 24 | 62% | 77%** | 78%*** | 37% | 74%†*** | 66%*** | 42% | 61%*** | 65%*** | 27% | 45%*** | 46%*** |
| Week 52 | 56% | 73%*** | 73%*** | 71%†† | 62% | |||||||
| ACR50 | ||||||||||||
| Week 12 | 33% | 55%*** | 60%*** | 17% | 45%††*** | 35%*** | 13% | 33%*** | 34%*** | 8% | 20%** | 28%*** |
| Week 24 | 43% | 60%** | 63%*** | 19% | 51%*** | 45%*** | 21% | 41%*** | 44%*** | 13% | 23%* | 29%*** |
| Week 52 | 38% | 57%*** | 62%*** | 56%† | 47% | |||||||
| ACR70 | ||||||||||||
| Week 12 | 16% | 31%*** | 34%*** | 5% | 19%†*** | 13%*** | 3% | 18%*** | 18%*** | 2% | 13%*** | 11%** |
| Week 24 | 21% | 42%*** | 40%*** | 8% | 30%†*** | 22%*** | 8% | 25%*** | 24%*** | 3% | 13%*** | 17%*** |
| Week 52 | 25% | 42%*** | 46%*** | 37% | 31% | |||||||
| DAS28-hsCRP ≤3.2 | ||||||||||||
| Week 12 | 30% | 47%*** | 56%*** | 14% | 44%††*** | 35%*** | 17% | 36%*** | 39%*** | 9% | 24%*** | 32%*** |
| Week 24 | 38% | 57%*** | 60%*** | 19% | 52%*** | 48%*** | 24% | 46%*** | 52%*** | 11% | 20%* | 33%*** |
| Week 52 | 38% | 57%*** | 63%*** | 56%† | 48% | |||||||
| SDAI ≤3.3 | ||||||||||||
| Week 12 | 6% | 14%* | 20%*** | 2% | 8%*** | 7%*** | 1% | 9%*** | 9%*** | 2% | 2% | 5% |
| Week 24 | 10% | 22%** | 23%*** | 3% | 16%*** | 14%*** | 4% | 17%*** | 15%*** | 2% | 5% | 9%** |
| Week 52 | 13% | 25%** | 30%*** | 23% | 18% | |||||||
| CDAI ≤2.8 | ||||||||||||
| Week 12 | 7% | 14%* | 19%*** | 2% | 8%*** | 7%** | 2% | 10%*** | 9%*** | 2% | 3% | 6% |
| Week 24 | 11% | 21%** | 22%** | 4% | 16%*** | 12%*** | 4% | 15%*** | 15%*** | 3% | 5% | 9%* |
| Week 52 | 16% | 25%* | 28%** | 22% | 18% | |||||||
| HAQ-DI Minimum Clinically Important Difference (decrease in HAQ-DI score of ≥0.30) | ||||||||||||
| Week 12 | 60% | 81%*** | 77%*** | 46% | 68%*** | 64%*** | 44% | 60%*** | 56%** | 35% | 48%* | 54%*** |
| Week 24 | 66% | 77%* | 74% | 37% | 67%†*** | 60%*** | 37% | 58%*** | 55%*** | 24% | 41%*** | 44%*** |
| Week 52 | 53% | 65%* | 67%** | 61% | 55% | |||||||
Note: Proportions of responders at each time point based on those initially randomised to treatment (N). Patients who discontinued or received rescue therapy were considered as non-responders thereafter.
Abbreviations: ADA = adalimumab; BARI = baricitinib; IR = inadequate responder; MTX = methotrexate; PBO = Placebo
* p≤0.05; p≤0.01; *p≤0.001 vs. placebo (vs. MTX for study RA-BEGIN)
† p≤0.05; ††p≤0.01; †††p≤0.001 vs. adalimumab
Radiographic response
The effect of baricitinib on progression of structural joint damage was evaluated radiographically in studies RA-BEGIN, RA-BEAM and RA-BUILD and assessed using the modified Total Sharp Score (mTSS) and its components, the erosion score and joint space narrowing score.
Treatment with baricitinib 4 mg resulted in a statistically significant inhibition of progression of structural joint damage (Table 5). Analyses of erosion and joint space narrowing scores were consistent with the overall scores. The proportion of patients with no radiographic progression (mTSS change ≤0) was significantly higher with baricitinib 4 mg compared to placebo at weeks 24 and 52.
Table 5. Radiographic changes:
| Study | RA-BEGIN MTX-naïve patients | RA-BEAM MTX-IR patients | RA-BUILD cDMARD-IR patients | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment group | MTX | BARI 4 mg | BARI 4 mg + MTX | PBOa | BARI 4 mg | ADA 40 mg Q2W | PBO | BARI 2 mg | BARI 4 mg |
| Modified Total Sharp Score, mean change from baseline | |||||||||
| Week 24 | 0.61 | 0.39 | 0.29* | 0.90 | 0.41*** | 0.33*** | 0.70 | 0.33* | 0.15** |
| Week 52 | 1.02 | 0.80 | 0.40** | 1.80 | 0.71*** | 0.60*** | |||
| Proportion of patients with no radiographic progressionb | |||||||||
| Week 24 | 68% | 76% | 81%** | 70% | 81%*** | 83%*** | 74% | 72% | 80% |
| Week 52 | 66% | 69% | 80%** | 70% | 79%** | 81%** | |||
Abbreviations: ADA = adalimumab; BARI = baricitinib; IR = inadequate responder;
MTX = methotrexate; PBO = Placebo
a Placebo data at week 52 derived using linear extrapolation
b No progression defined as mTSS change ≤0.
* p≤0.05; *p≤0.01; **p≤0.001 vs. placebo (vs. MTX for study RA-BEGIN)
Physical function response and health-related outcomes
Treatment with baricitinib 4 mg, alone or in combination with cDMARDs, resulted in a significant improvement in physical function (HAQ-DI) and pain (0-100 visual analogue scale) compared to all comparators (placebo, MTX, adalimumab). Improvements were seen as early as week 1 and, in studies RA-BEGIN and RA-BEAM, this was maintained for up to 52 weeks.
In RA-BEAM and RA-BUILD, treatment with baricitinib 4 mg resulted in a significant improvement in the mean duration and severity of morning joint stiffness compared to placebo or adalimumab as assessed using daily electronic patient diaries.
In all studies, baricitinib-treated patients reported improvements in patient-reported quality of life, as measured by the Short Form (36) Health Survey (SF-36) Physical Component Score and fatigue, as measured by the Functional Assessment of Chronic Illness Therapy-Fatigue score (FACIT-F).
Baricitinib 4 mg vs. 2 mg
Differences in efficacy between the 4 mg and the 2 mg doses were most notable in the bDMARD-inadequate responder (IR) population (RA-BEACON), in which statistically significant improvements in the ACR components of swollen joint count, tender joint count and ESR were shown for baricitinib 4 mg compared to placebo at week 24 but not for baricitinib 2 mg compared to placebo. In addition, for both study RA-BEACON and RA-BUILD, onset of efficacy was faster and the effect size was generally larger for the 4 mg dose groups compared to 2 mg.
In a long-term extension study, patients from Studies RA-BEAM, RA-BUILD and RA-BEACON who achieved sustained low disease activity or remission (CDAI ≤ 10) after at least 15 months of treatment with baricitinib 4 mg once daily were re-randomised 1:1 in a double-blind manner to continue 4 mg once daily or reduce dose to 2 mg once daily. The majority of patients maintained low disease activity or remission based on CDAI score:
- At week 12: 451/498 (91%) continuing 4 mg vs. 405/498 (81%) reduced to 2 mg (p≤0.001)
- At week 24: 434/498 (87%) continuing 4 mg vs. 372/498 (75%) reduced to 2 mg (p≤0.001)
- At week 48: 400/498 (80%) continuing 4 mg vs. 343/498 (69%) reduced to 2 mg (p≤0.001)
- At week 96: 347/494 (70%) continuing 4 mg vs. 297/496 (60%) reduced to 2 mg (p≤0.001)
The majority of patients who lost their low disease activity or remission status after dose reduction could regain disease control after the dose was returned to 4 mg.
Adults with atopic dermatitis
The efficacy and safety of baricitinib as monotherapy or in combination with topical corticosteroids (TCS) were assessed in 3 Phase III randomised, double-blind, placebo-controlled, 16 week studies (BREEZE-AD1, -AD2, and -AD7). The studies included 1 568 patients with moderate to severe atopic dermatitis defined by Investigator's Global Assessment (IGA) score ≥3, an Eczema Area and Severity Index (EASI) score ≥16, and a body surface area (BSA) involvement of ≥10%. Eligible patients were over 18 years of age and had previous inadequate response or were intolerant to topical medicinal products. Patients were permitted to receive rescue treatment (which included topical or systemic therapy), at which time they were considered non-responders. At baseline of study BREEZE-AD7, all patients were on concomitant topical corticosteroids therapy and patients were permitted to use topical calcineurin inhibitors. All patients who completed these studies were eligible to enrol in a long term extension study (BREEZE AD-3) for up to 2 years of continued treatment.
The Phase III randomised, double-blind, placebo-controlled BREEZE-AD4 study evaluated the efficacy of baricitinib in combination with topical corticosteroids over 52 weeks in 463 patients with moderate to severe atopic dermatitis with failure, intolerance, or contraindication to oral ciclosporin treatment.
Baseline characteristics
In the placebo-controlled Phase III studies (BREEZE-AD1, -AD2, -AD7, and -AD4), across all treatment groups, 37% were female, 64% were Caucasian, 31% were Asian and 0.6% were Black, and the mean age was 35.6 years. In these studies, 42% to 51% of patients had a baseline IGA of 4 (severe atopic dermatitis), and 54% to 79% of patients had received prior systemic treatment for atopic dermatitis. The baseline mean EASI score ranged from 29.6 to 33.5, the baseline weekly averaged Itch Numerical Rating Scale (NRS) ranged from 6.5 to 7.1, the baseline mean Dermatology Life Quality Index (DLQI) ranged from 13.6 to 14.9, and the baseline mean Hospital Anxiety and Depression Scale (HADS) Total score ranged from 10.9 to 12.1.
Clinical response
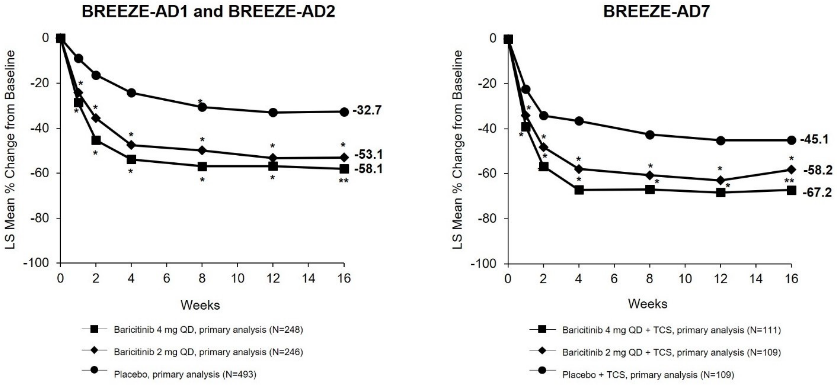
16-week monotherapy (BREEZE-AD1, -AD2) and TCS combination (BREEZE-AD7) studies A significantly larger proportion of patients randomised to baricitinib 4 mg achieved an IGA 0 or 1 response (primary outcome), EASI75, or an improvement of ≥4 points on the Itch NRS compared to placebo at week 16 (Table 6). Figure 1 shows the mean percent change from baseline in EASI up to week 16.
A significantly greater proportion of patients randomised to baricitinib 4 mg achieved a ≥4-point improvement in the Itch NRS compared to placebo (within the first week of treatment for BREEZE-AD1 and AD2, and as early as week 2 for BREEZE-AD7; p<0.002).
Treatment effects in subgroups (weight, age, gender, race, disease severity, and previous treatment, including immunosuppressants) were consistent with the results in the overall study population.
Table 6. Efficacy of baricitinib at week 16 (FASa):
| Monotherapy | TCS Combination | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | BREEZE-AD1 | BREEZE-AD2 | BREEZE-AD7 | ||||||
| Treatment Group | PBO | BARI 2 mg | BARI 4 mg | PBO | BARI 2 mg | BARI 4 mg | PBO + TCS | BARI 2 mg + TCS | BARI 4 mg + TCS |
| N | 249 | 123 | 125 | 244 | 123 | 123 | 109 | 109 | 111 |
| IGA 0 or 1, % respondersb,c | 4.8 | 11.4** | 16.8** | 4.5 | 10.6** | 13.8** | 14.7 | 23.9 | 30.6** |
| EASI-75, % respondersc | 8.8 | 18.7** | 24.8** | 6.1 | 17.9** | 21.1** | 22.9 | 43.1* | 47.7** |
| Itch NRS (≥4 point improvement), % respondersc,d | 7.2 | 12.0 | 21.5** | 4.7 | 15.1** | 18.7** | 20.2 | 38.1* | 44.0** |
BARI = Baricitinib; PBO = Placebo
* statistically significant vs placebo without adjustment for multiplicity; **statistically significant vs placebo with adjustment for multiplicity.
a Full analysis set (FAS) including all randomised patients.
b Responder was defined as a patient with IGA 0 or 1 ("clear" or "almost clear") with a reduction of ≥2 points on 0-4 IGA scale.
c Non-Responder Imputation: Patients who received rescue treatment or with missing data were considered as non-responders.
d Results shown in subset of patients eligible for assessment (patients with itch NRS ≥4 at baseline).
Figure 1. Mean percent change from baseline in EASI (FAS)a:
BARI = Baricitinib; PBO = Placebo
* statistically significant vs placebo without adjustment for multiplicity; **statistically significant vs placebo with adjustment for multiplicity.
a Full analysis set (FAS) including all randomised patients.
b Responder was defined as a patient with IGA 0 or 1 ("clear" or "almost clear") with a reduction of ≥2 points on 0-4 IGA scale.
c Non-Responder Imputation: Patients who received rescue treatment or with missing data were considered as non-responders.
d Results shown in subset of patients eligible for assessment (patients with itch NRS ≥4 at baseline).
Maintenance of response
To evaluate maintenance of response, 1 373 subjects treated with baricitinib for 16 weeks in BREEZE-AD1 (N=541), BREEZE-AD2 (N=540) and BREEZE-AD7 (N=292) were eligible to enrol in a long term extension study BREEZE-AD3. Data are available up to 68 weeks of cumulative treatment for patients from BREEZE-AD1 and BREEZE-AD2, and up to 32 weeks of cumulative treatment for patients from BREEZE-AD7. Continued response was observed in patients with at least some response (IGA 0, 1 or 2) after initiating baricitinib.
Quality of life/patient-reported outcomes in atopic dermatitis
In both monotherapy studies (BREEZE-AD1 and BREEZE-AD2) and in the concomitant TCS study (BREEZE-AD7), baricitinib 4 mg significantly improved patient-reported outcomes, including itch NRS, sleep (ADSS), skin pain (skin pain NRS), quality of life (DLQI) and symptoms of anxiety and depression (HADS) that were uncorrected for multiplicity, at 16 weeks compared to placebo (See Table 7).
Table 7. Quality of life/patient-reported outcomes results of baricitinib monotherapy and baricitinib in combination with TCS at week 16 (FAS)a:
| Monotherapy | TCS Combination | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | BREEZE-AD1 | BREEZE-AD2 | BREEZE-AD7 | ||||||
| Treatment group | PBO | BARI 2 mg | BARI 4 mg | PBO | BARI 2 mg | BARI 4 mg | PBO + TCS | BARI 2 mg + TCS | BARI 4 mg + TCS |
| N | 249 | 123 | 125 | 244 | 123 | 123 | 109 | 109 | 111 |
| ADSS Item 2 ≥2-point improvement, % respondersc,d | 12.8 | 11.4 | 32.7* | 8.0 | 19.6 | 24.4* | 30.6 | 61.5* | 66.7* |
| Change in Skin Pain NRS, mean(SE)b | -0.84 (0.24) | -1.58 (0.29) | -1.93** (0.26) | -0.86 (0.26) | -2.61** (0.30) | -2.49** (0.28) | -2.06 (0.23) | -3.22* (0.22) | -3.73* (0.23) |
| Change in DLQI, mean(SE)b | -2.46 (0.57) | -4.30* (0.68) | -6.76* (0.60) | -3.35 (0.62) | -7.44* (0.71) | -7.56* (0.66) | -5.58 (0.61) | -7.50* (0.58) | -8.89* (0.58) |
| Change in HADS, mean(SE)b | -1.22 (0.48) | -3.22* (0.58) | -3.56* (0.52) | -1.25 (0.57) | -2.82 (0.66) | -3.71* (0.62) | -3.18 (0.56) | -4.75* (0.54) | -5.12* (0.54) |
BARI = Baricitinib; PBO = Placebo
* statistically significant vs placebo without adjustment for multiplicity; **statistically significant vs placebo with adjustment for multiplicity.
a Full analysis set (FAS) including all randomised patients.
b Results shown are LS mean change from baseline (SE). Data collected after rescue therapy or after permanent medicinal product discontinuation were considered missing. LS means are from Mixed Model with Repeated Measures (MMRM) analyses.
c ADSS Item 2: Number of night time awakenings due to itch.
d Nonresponder imputation: patients who received rescue treatment or with missing data were considered as nonresponders. Results shown in subset of patients eligible for assessment (patients with ADSS Item 2 ≥2 at baseline).
Clinical response in patients with experience with or a contra-indication to ciclosporin treatment (BREEZE-AD4 study)
A total of 463 patients were enrolled, who had either failed (n=173), or had an intolerance (n=75), or contraindication (n=126) to oral ciclosporin. The primary endpoint was the proportion of patients achieving EASI-75 at week 16. The primary and some of the most important secondary endpoints at week 16 are summarised in Table 8.
Table 8. Efficacy of baricitinib in combination with TCSa at week 16 in BREEZE-AD4 (FAS)b:
| Study | BREEZE-AD4 | ||
|---|---|---|---|
| Treatment group | PBOa | BARI 2 mga | BARI 4 mga |
| N | 93 | 185 | 92 |
| EASI-75, % respondersc | 17.2 | 27.6 | 31.5** |
| IGA 0 or 1, % respondersc,e | 9.7 | 15.1 | 21.7* |
| Itch NRS (≥4 point improvement), % respondersc,f | 8.2 | 22.9* | 38.2** |
| Change in DLQI mean (SE)d | -4.95 (0.752) | -6.57 (0.494) | -7.95* (0.705) |
BARI = Baricitinib; PBO = Placebo
* statistically significant vs placebo without adjustment for multiplicity; **statistically significant vs placebo with adjustment for multiplicity.
a All patients were on concomitant topical corticosteroids therapy and patients were permitted to use topical calcineurin inhibitors.
b Full analysis set (FAS) includes all randomised patients.
c Non-Responder Imputation: Patients who received rescue treatment or with missing data were considered as non-responders.
d Data collected after rescue therapy or after permanent medicinal product discontinuation were considered missing. LS means are from Mixed Model with Repeated Measures (MMRM) analyses.
e Responder was defined as a patient with IGA 0 or 1 ("clear" or "almost clear") with a reduction of ≥2 points on 0-4 IGA scale.
f Results shown in subset of patients eligible for assessment (patients with itch NRS ≥4 at baseline).
Alopecia areata
The efficacy and safety of baricitinib once daily were assessed in one adaptive Phase II/III study (BRAVE-AA1) and one Phase III study (BRAVE-AA2). The Phase III portion of BRAVE-AA1 study and the Phase III BRAVE-AA2 study were randomised, double blind, placebo-controlled, 36-week studies with extension phases up to 200 weeks. In both phase III studies, patients were randomised to placebo, 2 mg or 4 mg baricitinib in a 2:2:3 ratio. Eligible patients were adults between 18 years and 60 years of age for male patients, and between 18 years and 70 years of age for female patients, with a current episode of more than 6 months of severe alopecia areata (hair loss encompassing ≥50% of the scalp). Patients with a current episode of more than 8 years were not eligible unless episodes of regrowth had been observed on the affected areas of the scalp over the past 8 years. The only permitted concomitant alopecia areata therapies were finasteride (or other 5 alpha reductase inhibitors), oral or topical minoxidil and bimatoprost ophthalmic solution for eyelashes, if at a stable dose at study entry.
Both studies assessed as primary outcome the proportion of subjects who achieved a SALT (Severity of Alopecia Tool) score of ≤20 (80% or more scalp coverage with hair) at week 36. Additionally, both studies evaluated clinician assessment of eyebrow and eyelash hair loss using a 4-point scale (ClinRO Measure for Eyebrow Hair Loss, ClinRO Measure for Eyelash Hair Loss).
Baseline characteristics
The Phase III portion of BRAVE-AA1 study and the Phase III BRAVE-AA2 study included 1 200 adult patients. Across all treatment groups, the mean age was 37.5 years, 61% of patients were female. The mean duration of alopecia areata from onset and the mean duration of current episode of hair loss were 12.2 and 3.9 years, respectively. The median SALT score across the studies was 96 (this equals 96% scalp hair loss), and approximately 44% of patients were reported as alopecia universalis. Across the studies, 69% of patients had significant or complete eyebrow hair loss at baseline and 58% had significant or complete eyelash hair loss, as measured by ClinRO Measures for eyebrow and eyelash scores of 2 or 3. Approximately 90% of patients had received at least one treatment for alopecia areata at some point before entering the studies, and 50% at least one systemic immunosuppressant. The use of authorised concomitant alopecia areata treatments was reported by only 4.3% of patients during the studies.
Clinical response
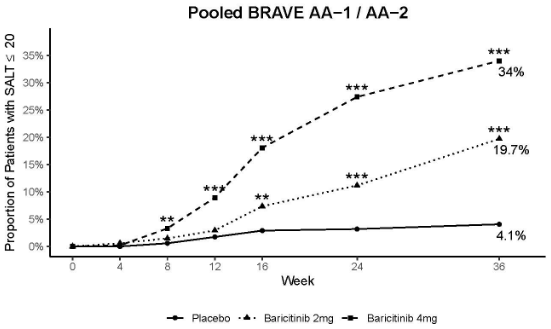
In both studies, a significantly greater proportion of patients randomised to baricitinib 4 mg once daily achieved a SALT ≤20 at week 36 compared to placebo, starting as early as week 8 in study BRAVE-AA1 and week 12 in study BRAVE-AA2. Consistent efficacy was seen across most of the secondary endpoints (Table 9). Figure 2 shows the proportion of patients achieving SALT ≤20 up to week 36.
Treatment effects in subgroups (gender, age, weight, eGFR, race, geographic region, disease severity, current alopecia areata episode duration) were consistent with the results in the overall study population at week 36.
Table 9. Efficacy of baricitinib through week 36 for pooled studies (Pooled Week 36 Efficacy Populationa):
| BRAVE-AA1 (phase III part of a phase II/III study) and BRAVE-AA2 (phase III study) Pooled Data* | |||
|---|---|---|---|
| Placebo N=345 | Baricitinib 2 mg N=340 | Baricitinib 4 mg N=515 | |
| SALT ≤20 at week 36 | 4.1% | 19.7%** | 34.0%** |
| SALT ≤20 at week 24 | 3.2% | 11.2% | 27.4%** |
| ClinRO Measure for Eyebrow Hair Loss of 0 or 1 at week 36 with a ≥2 point improvement from baselineb | 3.8% | 15.8% | 33.0%** |
| ClinRO Measure for Eyelash Hair Loss of 0 or 1 at week 36 with a ≥2 point improvement from baselineb | 4.3% | 12.0% | 33.9%** |
| Change in Skindex-16 adapted for alopecia areata emotions domain, mean (SE)c | -11.33 (1.768) | -19.89 (1.788) | -23.81 (1.488) |
| Change in Skindex-16 adapted for alopecia areata functioning domain, mean (SE)c | -9.26 (1.605) | -13.68 (1.623) | -16.93 (1.349) |
ClinRO = clinician-reported outcome; SE = standard error
a Pooled Week 36 Efficacy Population: All patients enrolled in the Phase III portion of Study BRAVE-AA1 and in Study BRAVE-AA2.
* The results of the pooled analysis are in line with those of the individual studies
** Statistically significant with adjustment for multiplicity in the graphical testing scheme within each individual study.
b Patients with ClinRO Measure for Eyebrow Hair loss score of ≥2 at baseline: 236 (Placebo), 240 (Baricitinib 2 mg), 349 (Baricitinib 4 mg). Patients with ClinRO Measure for Eyelash Hair loss score of ≥2 at baseline: 186 (Placebo), 200 (Baricitinib 2 mg), 307 (Baricitinib 4 mg). Both ClinRO Measures use a 4-point response scale ranging from 0 indicating no hair loss to 3 indicating no notable eyebrow/eyelashes hair.
c Sample sizes for analysis on Skindex-16 adapted for alopecia areata at week 36 are n= 256 (Placebo), 249 (Baricitinib 2 mg), 392 (Baricitinib 4 mg).
Figure 2. Proportion of patients with SALT ≤20 through week 36:
** p-value for baricitinib versus placebo ≤0.01; ***p-value for baricitinib versus placebo ≤0.001.
Efficacy up to week 52
The proportion of patients treated with baricitinib achieving a SALT ≤20 continued to increase after week 36, reaching 39.0% of patients on baricitinib 4 mg at week 52. The results for the baseline disease severity and episode duration subpopulations at week 52 were consistent with those observed at week 36 and with the results in the overall study population.
Dose tapering substudy
In the study BRAVE-AA2, patients who had received baricitinib 4 mg once daily since the initial randomization and achieved SALT ≤20 at week 52 were re-randomised in a double-blind manner to continue 4 mg once daily or reduce dose to 2 mg once daily. The results show that 96% of the patients who remained on baricitinib 4 mg and 74% of the patients who were re-randomised to baricitinib 2 mg maintained their response at week 76.
Juvenile idiopathic arthritis
The baricitinib clinical development programme for juvenile idiopathic arthritis consisted of one completed pivotal Phase III study (JUVE-BASIS) and one ongoing long-term open label safety extension study (JUVE-X).
JUVE-BASIS was a double-blind randomised withdrawal (DBW), up to 44-week placebo-controlled study to evaluate the efficacy and safety of baricitinib when administered once daily to patients from 2 years to less than 18 years of age with juvenile idiopathic arthritis who have had an inadequate response or intolerance to treatment with at least 1 conventional synthetic or biologic DMARD. This included patients with polyarticular juvenile idiopathic arthritis (rheumatoid factor positive or rheumatoid factor negative), extended oligoarticular course juvenile idiopathic arthritis, enthesitis-related juvenile idiopathic arthritis, and juvenile psoriatic arthritis as defined by the International League of Associations for Rheumatology (ILAR) criteria. Patients who participated in JUVE-BASIS were eligible for enrollment into study JUVE-X.
In JUVE-BASIS, patients received open-label once daily baricitinib for approximately 12 weeks from baseline. Patients 2 to less than 9 years received 2 mg daily and patients 9 to less than 18 years received 4 mg daily, to attain an equivalent exposure to a 4 mg dose in adults. At week 12, treatment response (based on PedACR30 criteria) was reviewed for each patient. Patients who achieved at least a PedACR30 response were randomised (1:1 ratio) to receive placebo or to remain on the same baricitinib dose in the 32-week double-blind, placebo-controlled phase. Patients who did not achieve PedACR30 were given the option of enrolling to JUVE-X.
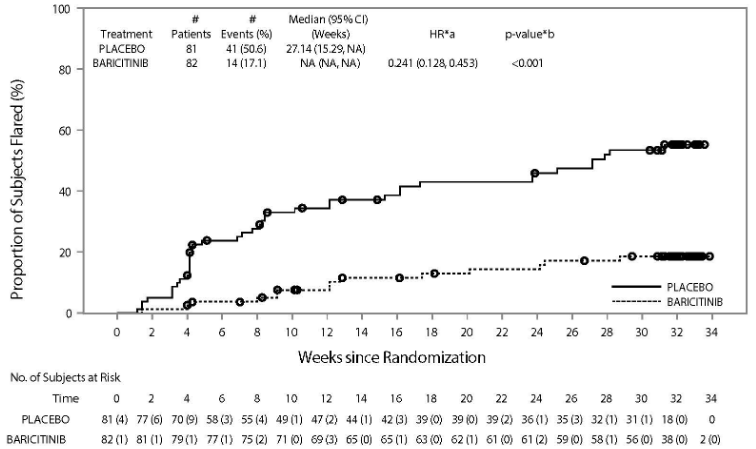
The primary efficacy endpoint of JUVE-BASIS was time to disease flare from the initiation of the DBW period to the end of the DBW period.
Baseline characteristics
A total of 220 patients enrolled JUVE-BASIS. Of these, 163 (74.4%) patients were eligible to be randomised into the DBW period to either baricitinib (n=82) or placebo (n=81). There were 144 patients with polyarticular juvenile idiopathic arthritis, 16 with extended oligoarticular course juvenile idiopathic arthritis, 50 with enthesitis-related juvenile idiopathic arthritis and 10 with juvenile psoriatic arthritis.
In JUVE-BASIS, the mean age was 13 years (standard deviation 3.0) and 69.1% were female. Patient numbers per age group were as follows: 2 to <6 years: n=6; 6 to <9 years: n=9; 9 to <12 years: n=30; and 12 to <18 years: n=175.
The average time reported by all patients in the study since juvenile idiopathic arthritis diagnosis was 4 years. Use of concomitant therapies was similar across treatment groups in the DBW period (most common concomitant csDMARDs included MTX, sulfasalazine and leflunomide). A total of 127 (57.7%) patients were on MTX at baseline.
Clinical response
In JUVE-BASIS, the group of baricitinib treated patients had a significantly longer time to disease flare compared to those receiving placebo (Figure 3). In addition, more patients treated with baricitinib achieved a PedACR value of 30/50/70/90/100 throughout the DBW period, as compared to placebo.
Figure 3. Time to disease flare during the DBW period:
CI = confidence interval; HR = hazard ratio; NA = not applicable; No. = number
*a HR-stratified by juvenile idiopathic arthritis categories (polyarticular and extended oligoarticular versus enthesitis-related arthritis and juvenile psoriatic arthritis).
*b P-value is from logrank test stratified by juvenile idiopathic arthritis categories (polyarticular and extended oligoarticular versus enthesitis-related arthritis and juvenile psoriatic arthritis).
Time to disease flare and PedACR score results were overall consistent across juvenile idiopathic arthritis subtypes and background characteristics (including age, geography, weight, prior use of biologic, concomitant use of MTX or corticosteroids), and were consistent with those for the overall study population.
Paediatric atopic dermatitis
The efficacy and safety of baricitinib in combination with TCS were assessed in a single Phase III randomised, double-blind, placebo-controlled, 16 week study (BREEZE-AD-PEDS). The study included 483 patients with moderate to severe atopic dermatitis defined by IGA score ≥3, an EASI score ≥16, and a BSA involvement of ≥10%. Eligible patients were 2 to less than 18 years of age and had previous inadequate response or were intolerant to topical medications and were candidates for systemic therapy. All patients were prescribed concomitant low or medium potency topical corticosteroids and patients were permitted to use topical calcineurin inhibitors during the study. Patients were randomised to placebo or baricitinib low, medium or high dose tested (resulting in equivalent exposure to 1 mg, 2 mg or 4 mg in adult AD patients, respectively) in a 1:1:1:1 ratio. The study includes an ongoing long-term extension for up to 4 years.
Baseline characteristics
Across all treatment groups, 76% were Caucasian, 15% were Asian and 3% were Black, 50% were female and mean age was 12 years with 72% at least 10 years of age and 28% less than 10 years of age. Patients 6 years and younger comprised 14% of the population (6 years [N=28], 5 years [N=11], 4 years [N=16], 3 years [N=8], 2 years [N=5]). In this study, 38% of patients had a baseline IGA of 4 (severe atopic dermatitis), and 42% of patients had received prior systemic treatment for atopic dermatitis. The baseline EASI score ranged from 12.2 to 70.8, the baseline weekly averaged Itch Numeric Rating Scale (NRS) in patients at least 10 years of age was 5.5 (SD=2.6).
Clinical response
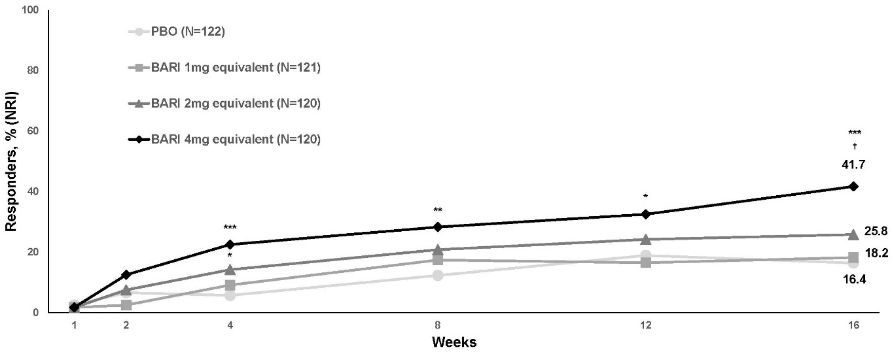
A statistically significant larger proportion of patients randomised to the baricitinib 4 mg equivalent dose achieved an IGA 0 or 1 response (primary outcome), EASI75, or an improvement of ≥4 points on the Itch NRS compared to placebo at week 16 (Table 10). Figure 4 shows the time course of achieving IGA 0 or 1.
Treatment effects in subgroups (weight, age, gender, race, disease severity, and previous treatment, including immunosuppressants) were consistent with the results in the overall study population.
Table 10. Efficacy of baricitinib in paediatric patients at week 16a:
| Study | BREEZE-AD-PEDS | |
|---|---|---|
| Treatment group | PBO | BARI 4 mg equivalent |
| N | 122 | 120 |
| IGA 0 or 1, % respondersb,c | 16.4 | 41.7** |
| EASI75, % respondersc | 32.0 | 52.5** |
| Itch NRS (≥4 point improvement), % respondersc,d | 16.4 | 35.5** |
BARI = Baricitinib; PBO = Placebo
** Statistically significant vs placebo with adjustment for multiplicity.
a Intent to Treat (ITT) population (all randomized patients)
b Responder was defined as a patient with IGA 0 or 1 ("clear" or "almost clear") with a reduction of ≥2 points on 0-4 IGA scale.
c Non-Responder Imputation: Patients who received rescue treatment or with missing data were considered as non-responders.
d Results shown in subset of patients eligible for assessment (patients aged ≥10 years with Itch NRS ≥4 at baseline, BARI 4 mg equivalent N=62; Placebo, N=55).
Figure 4. Time course for achieving IGA 0 or 1 with ≥2 points improvement in paediatric patients through week 16:
BARI = baricitinib; NRI = non-responder imputation; PBO = placebo
* p<0.05; *p<0.01; **p<0.001 vs. PBO (nominal p-value; logistic regression analysis); †Statistically significant with multiplicity adjustment
A significantly greater proportion of patients randomised to the baricitinib 4 mg equivalent dose achieved a ≥4-point improvement in the Itch NRS compared to placebo as early as week 4 (adjusted for multiplicity).
The need for concomitant TCS use was reduced as demonstrated by a median reduction in gram quantity of TCS use for the baricitinib 4 mg equivalent dose versus placebo over 16 weeks and a greater median number of TCS-free days for the baricitinib 4 mg equivalent dose (25 days) versus placebo (11 days) over 16 weeks.
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with baricitinib in one or more subsets of the paediatric population in chronic idiopathic arthritis and alopecia areata (see section 4.2 for information on paediatric use).
The efficacy of baricitinib up to 12 mg/day has been evaluated in 71 patients with CANDLE (chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature, n=10), CANDLE-related conditions (CANDLE-RC, n=9), SAVI (Stimulator of interferon gene-Associated Vasculopathy with onset during Infancy, n=8), Juvenile DermatoMyositis (JDM, n=5), and Aicardi-Goutières syndrome (AGS, n=39). Total patient-years of exposure (PYE) was 251. Due to methodological insufficiencies no definite conclusion could be drawn on the efficacy of baricitinib in these patients. Although safety patterns showed similarities with the adult indications, frequencies of adverse events were generally higher. Three deaths were observed, in the AGS population; it is unclear whether these deaths were related to treatment with baricitinib.
The efficacy and safety of baricitinib were evaluated in 29 patients from 2 to <18 years of age with active JIA-associated uveitis or chronic anterior antibody positive uveitis. MTX-IR (n=10) were assigned to baricitinib (n=5) or adalimumab (n=5); bDMARD-IR (n=19) were all assigned to baricitinib. Baricitinib was dosed 2 mg once daily for patients 2 to <9 years old and 4 mg once daily for those 9 to <18 years old, adalimumab dosing was 20 mg (if <30 kg), or 40 mg (if ≥30 kg) once every two weeks.
The primary endpoint was the proportion of patients with a 2 step decrease in the level of inflammation (anterior chamber cells) according to the SUN (standardisation of uveitis nomenclature) criteria or decrease to zero through week 24, in the eye most severely affected at baseline. Eight (33.3%) patients were baricitinib responders (7 bDMARD-IR and 1 MTX-IR), but the response rate between the two cohorts did not show a statistical significance.
Pharmacokinetic properties
Following oral administration of baricitinib, a dose-proportional increase in systemic exposure was observed in the therapeutic dose range. The PK of baricitinib is linear with respect to time.
Absorption
Following oral administration, baricitinib is rapidly absorbed with a median tmax of approximately 1 hour (range 0.5-3.0 h) and an absolute bioavailability of approximately 79% (CV=3.94%). Food intake led to a decreased exposure by up to 14%, a decrease in Cmax by up to 18% and delayed tmax by 0.5 hours. Administration with meals was not associated with a clinically relevant effect on exposure.
Distribution
Mean volume of distribution following intravenous infusion administration was 76 L, indicating distribution of baricitinib into tissues. Baricitinib is approximately 50% bound to plasma proteins.
Biotransformation
Baricitinib metabolism is mediated by CYP3A4, with less than 10% of the dose identified as undergoing biotransformation. No metabolites were quantifiable in plasma. In a clinical pharmacology study, baricitinib was excreted predominately as the unchanged active substance in urine (69%) and faeces (15%) and only 4 minor oxidative metabolites were identified (3 in urine; 1 in faeces) constituting approximately 5% and 1% of the dose, respectively. In vitro, baricitinib is a substrate for CYP3A4, OAT3, Pgp, BCRP and MATE2-K, and may be a clinically relevant inhibitor of the transporter OCT1 (see section 4.5). Baricitinib is not an inhibitor of the transporters OAT1, OAT2, OAT3, OCT2, OATP1B1, OATP1B3, BCRP, MATE1 and MATE2-K at clinically relevant concentrations.
Elimination
Renal elimination is the principal mechanism for baricitinib's clearance through glomerular filtration and active secretion via OAT3, Pgp, BCRP and MATE2-K. In a clinical pharmacology study, approximately 75% of the administered dose was eliminated in the urine, while about 20% of the dose was eliminated in the faeces.
Mean apparent clearance (CL/F) and half-life in patients with rheumatoid arthritis was 9.42 L/hr (CV=34.3%) and 12.5 hrs (CV=27.4%), respectively. Cmax and AUC at steady state are 1.4- and 2.0-fold higher, respectively, in subjects with rheumatoid arthritis compared to healthy subjects.
Mean apparent clearance (CL/F) and half-life in patients with atopic dermatitis was 11.2 L/hr (CV=33.0%) and 12.9 hrs (CV=36.0%), respectively. Cmax and AUC at steady state in patients with atopic dermatitis are 0.8-fold those seen in rheumatoid arthritis.
Mean apparent clearance (CL/F) and half-life in patients with alopecia areata was 11.0 L/hr (CV=36.0%) and 15.8 hrs (CV=35.0%), respectively. Cmax and AUC at steady state in patients with alopecia areata are 0.9-fold those seen in rheumatoid arthritis.
Renal impairment
Renal function was found to significantly affect baricitinib exposure. The mean ratios of AUC in patients with mild and moderate renal impairment to patients with normal renal function are 1.41 (90% CI: 1.15-1.74) and 2.22 (90% CI: 1.81-2.73), respectively. The mean ratios of Cmax in patients with mild and moderate renal impairment to patients with normal renal function are 1.16 (90%CI: 0.92-1.45) and 1.46 (90%CI: 1.17-1.83), respectively. See section 4.2 for dose recommendations.
Hepatic impairment
There was no clinically relevant effect on the PK of baricitinib in patients with mild or moderate hepatic impairment. The use of baricitinib has not been studied in patients with severe hepatic impairment.
Elderly
Age ≥65 years or ≥75 years has no effect on baricitinib exposure (Cmax and AUC).
Paediatric population
Pharmacokinetics in paediatric patients with juvenile idiopathic arthritis
The half-life in paediatric patients from 2 to less than18 years was 8 to 9 hours.
Exposure in paediatric patients weighing <30 kg and ≥30 kg:
In patients <30 kg with a mean age and range of 8.1 (2.0-16.0) years, the mean and CV% for AUC and Cmax was 381 h*ng/mL (76%) and 62.1 ng/mL (39%), respectively. In patients ≥30 kg with mean age and range of 14.1 (9.0–17.0), the mean and CV% for AUC and Cmax was 438 h*ng/mL (68%) and 60.7 ng/mL (30%), respectively.
Exposure in paediatric patients weighing 10 to <20 kg and 20 to <30 kg:
In patients 10 to <20 kg with a mean age and range of 5.1 (2.0-8.0) years, the mean and CV% for AUC and Cmax was 458 h*ng/mL (81%) and 77.6 ng/mL (38%), respectively. In patients 20 to <30 kg with mean age and range of 10.3 (6.0–16.0), the mean and CV% for AUC and Cmax was 327 h*ng/mL (66%) and 51.2 ng/mL (22%), respectively.
Pharmacokinetics in paediatric patients with atopic dermatitis
The mean half-life in paediatric patients from 2 to less than 18 years was 13 to 18 hours.
Exposure in paediatric patients weighing <30 kg and ≥30 kg:
In patients <30 kg with a mean age and range of 6.4 (2.0-11.1) years, the mean and CV% for AUC and Cmax was 404 h*ng/mL (78%) and 60.4 ng/mL (28%), respectively. In patients ≥30 kg with mean age and range of 13.5 (6.2–17.9), the mean and CV% for AUC and Cmax was 529 h*ng/mL (102%) and 57.0 ng/mL (42%), respectively.
Exposure in paediatric patients weighing 10 to <20 kg and 20 to <30 kg:
In patients 10 to <20 kg with a mean age and range of 4.8 (2.0-6.9) years, the mean and CV% for AUC and Cmax was 467 h*ng/mL (80%) and 73.4 ng/mL (21%), respectively. In patients 20 to <30 kg with mean age and range of 7.5 (4.8–11.1), the mean and CV% for AUC and Cmax was 363 h*ng/mL (72%) and 52.0 ng/mL (21%), respectively
Other intrinsic factors
Body weight, age, sex, race, and ethnicity did not have a clinically relevant effect on the PK of baricitinib in adult patients. The mean effects of intrinsic factors on PK parameters (AUC and Cmax) were generally within the inter-subject PK variability of baricitinib. Therefore, no dose adjustment is needed based on these patient factors.
Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, genotoxicity and carcinogenic potential.
Decreases in lymphocytes, eosinophils and basophils as well as lymphoid depletion in organs/tissues of the immune system were observed in mice, rats and dogs. Opportunistic infections related to demodicosis (mange) were observed in dogs at exposures approximately 7 times the human exposure. Decreases in red blood cell parameters were observed in mice, rats and dogs at exposures approximately 6 to 36 times the human exposure. Degeneration of the sternal growth plate was observed in some dogs, at low incidence and also in control animals, but with a dose-effect relationship regarding severity. At present it is not known whether this is clinically relevant.
In rat and rabbit reproductive toxicology studies, baricitinib was shown to reduce foetal growth/weight and produce skeletal malformations (at exposures of approximately 10 and 39 times the human exposure, respectively). No adverse foetal effects were observed at exposures 2 times the human exposure based on AUC.
In a combined male/female rat fertility study, baricitinib decreased overall mating performance (decreased fertility and conception indices). In female rats there were decreased numbers of corpora lutea and implantation sites, increased pre-implantation loss, and/or adverse effects on intrauterine survival of the embryos. Since there were no effects on spermatogenesis (as assessed by histopathology) or semen/sperm endpoints in male rats, the decreased overall mating performance was likely the result of these female effects.
Baricitinib was detected in the milk of lactating rats. In a pre- and postnatal development study, decreased pup weights and decreased postnatal survival were observed at exposures 4 and 21 times, respectively, the human exposure.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.