PADCEV Powder for solution for infusion Ref.[49907] Active ingredients: Enfortumab vedotin
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Astellas Pharma Europe B.V., Sylviusweg 62, 2333 BE Leiden, The Netherlands
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, other antineoplastic agents, monoclonal antibodies
ATC code: L01FX13
Mechanism of action
Enfortumab vedotin is an antibody drug conjugate (ADC) targeting Nectin-4, an adhesion protein located on the surface of the urothelial cancer cells. It is comprised of a fully human IgG1-kappa antibody conjugated to the microtubule-disrupting agent MMAE via a protease-cleavable maleimidocaproyl valine-citrulline linker. Nonclinical data suggest that the anticancer activity of enfortumab vedotin is due to the binding of the ADC to Nectin-4-expressing cells, followed by internalisation of the ADC-Nectin-4 complex, and the release of MMAE via proteolytic cleavage. Release of MMAE disrupts the microtubule network within the cell, subsequently inducing cell cycle arrest, apoptosis, and immunogenic cell death. MMAE released from enfortumab vedotin targeted cells can diffuse into nearby Nectin-4 low-expressing cells resulting in cytotoxic cell death. Combination of enfortumab vedotin with PD-1 inhibitors results in enhanced anti-tumour activity, consistent with the complementary mechanisms of MMAE induced cell cytotoxicity and induction of immunogenic cell death, plus the up-regulation of immune function by PD-1 inhibition.
Cardiac electrophysiology
At the recommended dose of 1.25 mg/kg, enfortumab vedotin did not prolong the mean QTc interval to any clinically relevant extent based on ECG data from a study in patients with advanced urothelial cancer.
Clinical efficacy and safety
Enfortumab vedotin in combination with pembrolizumab
Previously untreated locally advanced or metastatic urothelial cancer
EV-302 (KEYNOTE-A39):
The efficacy of Padcev in combination with pembrolizumab was evaluated in study EV-302 (KEYNOTE-A39), an open-label, randomised, phase 3, multicentre study that enrolled 886 patients with unresectable or metastatic urothelial cancer who had not received prior systemic therapy for locally advanced or metastatic disease. Patients that received neoadjuvant chemotherapy or patients that received adjuvant chemotherapy following cystectomy were included in the study if recurrence was >12 months from completion of therapy. Patients were considered cisplatin-ineligible if they had at least one of the following criteria: glomerular filtration rate (GFR) between 30-59 mL/min, Eastern Cooperative Oncology Group (ECOG) performance status ≥2, Grade ≥2 hearing loss or New York Heart Association (NYHA) Class III heart failure.
Patients were randomised 1:1 to receive either enfortumab vedotin in combination with pembrolizumab (arm A) or gemcitabine and platinum-based chemotherapy (cisplatin or carboplatin) (arm B). Patients in arm A received enfortumab vedotin 1.25 mg/kg as an intravenous infusion over 30 minutes on Days 1 and 8 of a 21-day cycle followed by pembrolizumab 200 mg on Day 1 of a 21-day cycle approximately 30 minutes after enfortumab vedotin. Patients in arm B received gemcitabine
1000 mg/m² administered on Days 1 and 8 of a 21-day cycle with cisplatin 70 mg/m² or carboplatin (AUC = 4.5 or 5 mg/mL/min according to local guidelines) administered on Day 1 of a 21-day cycle. Treatment was continued until disease progression, unacceptable toxicity or completion of the maximum number of treatment cycles (chemotherapy, 6 cycles; pembrolizumab, 35 cycles; enfortumab vedotin, no set maximum).
Patients randomised to the gemcitabine and platinum-based chemotherapy arm were permitted to receive maintenance immunotherapy (e.g., avelumab). Randomisation was stratified by cisplatin eligibility (eligible versus ineligible), PD-L1 expression (CPS≥10 versus CPS<10), and presence of liver metastases (present versus absent). PD-L1 expression was based on the PD-L1 IHC 22C3 pharmDx kit.
Patients were excluded from the study if they had active CNS metastases, ongoing sensory or motor neuropathy Grade ≥2, uncontrolled diabetes defined as haemoglobin A1C (HbA1c) ≥8% or HbA1c ≥7% with associated diabetes symptoms, autoimmune disease or a medical condition that required immunosuppression, pneumonitis or other forms of interstitial lung disease.
The median age was 69 years (range: 22 to 91); 77% were male; and most were White (67%) or Asian (22%). Patients had a baseline ECOG performance status of 0 (49%), 1 (47%) or 2 (3%). Forty-seven percent of patients had a documented baseline HbA1c of <5.7%. At baseline, 95% of patients had metastatic urothelial cancer and 5% of patients had unresectable urothelial cancer. Seventy-two percent of patients had visceral metastasis at baseline including 22% with liver metastases. Eighty-five percent of patients had urothelial carcinoma (UC) histology, 6% had UC mixed squamous differentiation and 2% had UC mixed other histologic variants. Forty-six percent of patients were cisplatin-ineligible and 54% were cisplatin-eligible at time of randomisation. Of the 877 patients tested who had tissue evaluable for PD-L1 expression, 58% of patients had tumours that expressed PD-L1 with a CPS ≥10 and 42% had tumours that expressed PD-L1 with a CPS <10. The median follow-up time was 17.3 months (range: 0.3 to 37.2).
The primary efficacy outcome measures were Overall Survival (OS) and Progression Free Survival (PFS) as assessed by BICR according to RECIST v1.1. Secondary efficacy outcome measures included Objective Response Rate (ORR) as assessed by BICR according to RECIST v1.1.
The study showed statistically significant improvements in OS, PFS and ORR for patients randomised to enfortumab vedotin in combination with pembrolizumab as compared to gemcitabine and platinum-based chemotherapy.
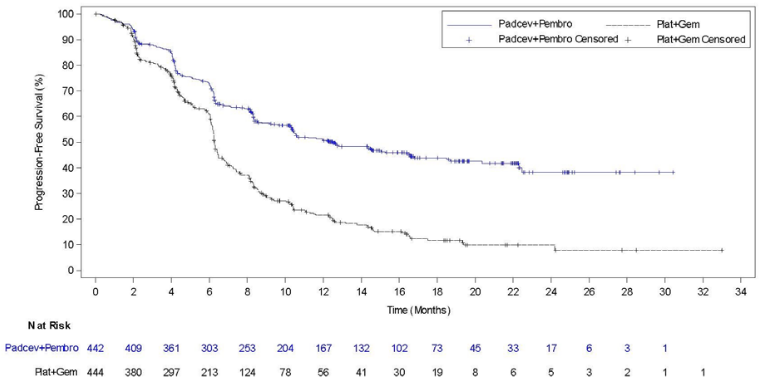
Table 4, Figures 1 and 2 summarise the efficacy results for EV-302.
Table 4. Efficacy Results in EV-302:
| Endpoint | Padcev + pembrolizumab n=442 | Gemcitabine+ platinum n=444 |
|---|---|---|
| Overall Survival | ||
| Number (%) of patients with events | 133 (30.1) | 226 (50.9) |
| Median in months (95% CI)a | 31.5 (25.4, -) | 16.1 (13.9, 18.3) |
| Hazard ratiob (95% CI) | 0.468 (0.376, 0.582) | |
| 2-sided p-valuec | <0.00001 | |
| Progression Free Survivald | ||
| Number (%) of patients with events | 223 (50.5) | 307 (69.1) |
| Median in months (95% CI)a | 12.5 (10.4, 16.6) | 6.3 (6.2, 6.5) |
| Hazard ratiob (95% CI) | 0.450 (0.377, 0.538) | |
| 2-sided p-valuec | <0.00001 | |
| Objective Response Rate (CR + PR)d,f | ||
| Confirmed ORR () (95 CI)e | 67.7 (63.1, 72.1) | 44.4 (39.7, 49.2) |
| 2-sided p-valueg | <0.00001 | |
| Duration of Responsed,f | ||
| Median in months (95% CI)a | NR (20.2, -) | 7.0 (6.2, 10.2) |
NR = Not reached.
a Based on the complementary log-log transformation method (Collett, 1994).
b Based on stratified Cox proportional hazards model. A hazard ratio <1 favors the enfortumab vedotin in combination with pembrolizumab arm.
c Based on stratified log-rank test.
d Evaluated by BICR using RECIST v1.1
e Based on the Clopper-Pearson method (Clopper 1934).
f Includes only patients with measurable disease at baseline (n=437 for enfortumab vedotin in combination with pembrolizumab, n=441 for gemcitabine plus platinum). The duration of response was estimated for responders.
g Based on Cochran-Mantel-Haenszel test stratified by PD-L1 expression, cisplatin eligibility and liver metastases
Figure 1. Kaplan Meier plot of overall survival, EV-302:
Figure 2. Kaplan Meier plot of progression-free survival, EV-302
Enfortumab vedotin as monotherapy
Previously treated locally advanced or metastatic urothelial cancer
EV-301
The efficacy of Padcev as monotherapy was evaluated in study EV-301, an open-label, randomised, phase 3, multicentre study that enrolled 608 patients with locally advanced or metastatic urothelial cancer who have previously received a platinum-containing chemotherapy and a programmed death receptor 1 (PD-1) or programmed death ligand 1 (PD-L1) inhibitor. The primary endpoint of the study was Overall Survival (OS) and secondary endpoints included Progression Free Survival (PFS) and Objective Response Rate (ORR) [PFS and ORR were evaluated by investigator assessment using RECIST v1.1]. Patients were randomised 1:1 to receive either enfortumab vedotin 1.25 mg/kg on Days 1, 8 and 15 of a 28-day cycle, or one of the following chemotherapies as decided by the investigator: docetaxel 75 mg/m² (38%), paclitaxel 175 mg/m² (36%) or vinflunine 320 mg/m² (25%) on Day 1 of a 21-day cycle.
Patients were excluded from the study if they had active CNS metastases, ongoing sensory or motor neuropathy ≥ Grade 2, known history of human immunodeficiency virus (HIV) infection (HIV 1 or 2), active Hepatitis B or C, or uncontrolled diabetes defined as HbA1c ≥8% or HbA1c ≥7% with associated diabetes symptoms.
The median age was 68 years (range: 30 to 88 years), 77% were male, and most patients were White (52%) or Asian (33%). All patients had a baseline ECOG performance status of 0 (40%) or 1 (60%). Ninety-five percent (95%) of patients had metastatic disease and 5% had locally advanced disease. Eighty percent of patients had visceral metastases including 31% with liver metastases. Seventy-six percent of patients had urothelial carcinoma/transitional cell carcinoma (TCC) histology, 14% had urothelial carcinoma mixed and approximately 10% had other histologic variants. A total of 76 (13%) patients had received ≥3 lines of prior systemic therapy. Fifty-two percent (314) of patients had received prior PD-1 inhibitor, 47% (284) had received prior PD-L1 inhibitor, and an additional 1% (9) patients had received both PD-1 and PD-L1 inhibitors. Only 18% (111) of patients had a response to prior therapy with a PD-1 or PD-L1 inhibitor. Sixty-three percent (383) of patients had received prior cisplatin- based regimens, 26% (159) had received prior carboplatin-based regimens, and an additional 11% (65) had received both cisplatin and carboplatin- based regimens.
Table 5 summarises the efficacy results for the EV – 301 study, after a median follow-up time of 11.1 months (95% CI: 10.6 to 11.6).
Table 5. Efficacy results in EV-301:
| Endpoint | Padcev N=301 | Chemotherapy N=307 |
|---|---|---|
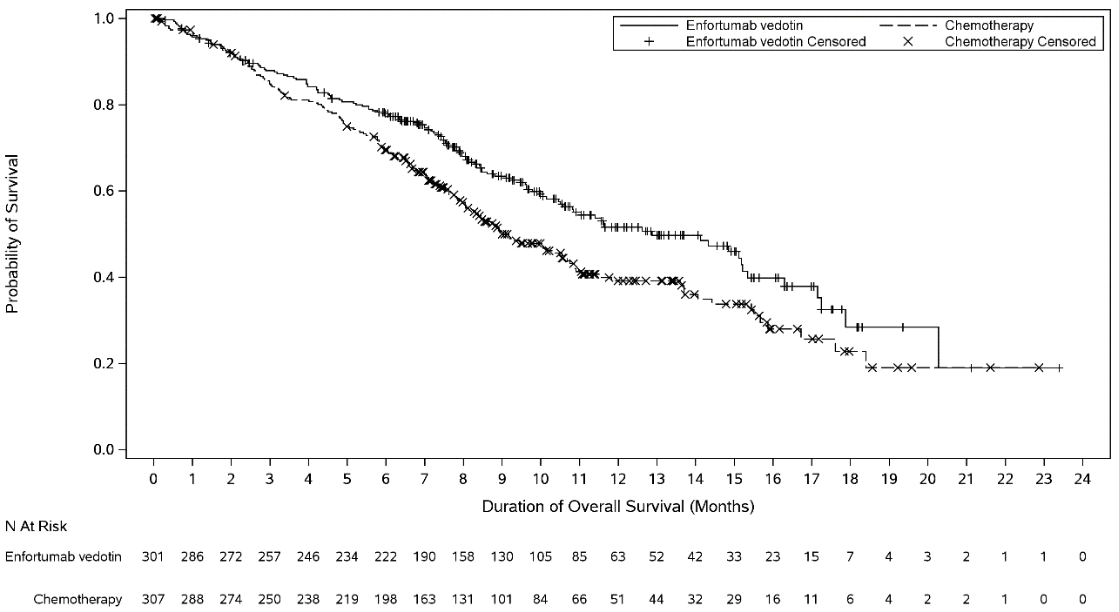
| Overall Survival | ||
| Number (%) of patients with events | 134 (44.5) | 167 (54.4) |
| Median in months (95% CI) | 12.9 (10.6, 15.2) | 9.0 (8.1, 10.7) |
| Hazard ratio (95% CI) | 0.702 (0.556, 0.886) | |
| 1-sided p-value | 0.00142* | |
| Progression Free Survival† | ||
| Number (%) of patients with events | 201 (66.8) | 231 (75.2) |
| Median in months (95% CI) | 5.6 (5.3, 5.8) | 3.7 (3.5, 3.9) |
| Hazard ratio (95% CI) | 0.615 (0.505, 0.748) | |
| 1-sided p-value | <0.00001‡ | |
| Objective Response Rate (CR + PR)† | ||
| ORR (%) (95% CI) | 40.6 (35.0, 46.5) | 17.9 (13.7, 22.8) |
| 1-sided p-value | <0,001§ | |
| Complete response rate (%) | 4.9 | 2.7 |
| Partial response rate (%) | 35.8 | 15.2 |
| Duration of Response for responders | ||
| Median in months (95% CI) | 7.4 (5.6, 9.5) | 8.1 (5.7, 9.6) |
* pre-determined efficacy boundary = 0.00679, 1-sided (adjusted by observed deaths of 301)
† evaluated by investigator assessment using RECIST v1.1
‡ pre-determined efficacy boundary = 0.02189, 1-sided (adjusted by observed PFS1 events of 432)
§ pre-determined efficacy boundary = 0.025, 1-sided (adjusted by 100% information fraction)
Figure 3. Kaplan Meier plot of overall survival:
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with enfortumab vedotin in all subsets of the paediatric population in urothelial cancer (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Distribution
The mean estimate of steady-state volume of distribution of ADC was 12.8 L following 1.25 mg/kg of enfortumab vedotin. In vitro, the binding of unconjugated MMAE to human plasma proteins ranged from 68% to 82%. Unconjugated MMAE is not likely to displace or to be displaced by highly protein-bound medicinal products. In vitro studies indicate that unconjugated MMAE is a substrate of P-glycoprotein.
Biotransformation
A small fraction of unconjugated MMAE released from enfortumab vedotin is metabolised. In vitro data indicate that the metabolism of unconjugated MMAE occurs primarily via oxidation by CYP3A4.
Elimination
The mean clearance of ADC and unconjugated MMAE in patients was 0.11 L/h and 2.11 L/h, respectively. ADC elimination exhibited a multi-exponential decline with a half-life of 3.6 days. Elimination of unconjugated MMAE appeared to be limited by its rate of release from enfortumab vedotin. Unconjugated MMAE elimination exhibited a multi-exponential decline with a half-life of 2.6 days.
Excretion
The excretion of unconjugated MMAE occurs mainly in faeces with a smaller proportion in urine. After a single dose of another ADC that contained unconjugated MMAE, approximately 24% of the total unconjugated MMAE administered was recovered in faeces and urine as unchanged unconjugated MMAE over a 1-week period. The majority of recovered unconjugated MMAE was excreted in faeces (72%). A similar excretion profile is expected for unconjugated MMAE after enfortumab vedotin administration.
Special populations
Elderly
Population pharmacokinetic analysis indicates that age [range: 24 to 90 years; 60% (450/748) >65 years, 19% (143/748) >75 years] does not have a clinically meaningful effect on the pharmacokinetics of enfortumab vedotin.
Race and gender
Based on population pharmacokinetic analysis, race [69% (519/748) White, 21% (158/748) Asian, 1% (10/748) Black and 8% (61/748) others or unknown] and gender [73% (544/748) male] do not have a clinically meaningful effect on the pharmacokinetics of enfortumab vedotin.
Renal impairment
The pharmacokinetics of ADC and unconjugated MMAE were evaluated after the administration of 1.25 mg/kg of enfortumab vedotin to patients with mild (CrCL >60–90 mL/min), moderate (CrCL 30–60 mL/min) and severe (CrCL 15–<30 mL/min) renal impairment. No significant differences in AUC exposure of ADC or unconjugated MMAE were observed in patients with mild, moderate or severe renal impairment compared to patients with normal renal function. Enfortumab vedotin has not been evaluated in patients with end stage renal disease (CrCL <15 mL/min).
Hepatic impairment
Based on population pharmacokinetics analysis using data from clinical studies in patients with metastatic UC, there was no significant differences in ADC exposure and a 37% and 16% increase in unconjugated MMAE average concentrations in patients with previously treated and previously untreated locally advanced or metastatic urothelial cancer, respectively, with mild hepatic impairment (total bilirubin of 1 to 1.5 × ULN and AST any, or total bilirubin ≤ ULN and AST > ULN) compared to patients with normal hepatic function. Enfortumab vedotin has only been studied in a limited number of patients with moderate hepatic impairment (n=5) or severe hepatic impairment (n=1). The effect of moderate or severe hepatic impairment (total bilirubin >1.5 x ULN and AST any) or liver transplantation on the pharmacokinetics of ADC or unconjugated MMAE is unknown.
Physiologically based pharmacokinetic modeling predictions
Concomitant use of enfortumab vedotin with ketoconazole (a combined P-gp and strong CYP3A inhibitor) is predicted to increase unconjugated MMAE Cmax and AUC exposure to a minor extent, with no change in ADC exposure.
Concomitant use of enfortumab vedotin with rifampin (a combined P-gp and strong CYP3A inducer) is predicted to decrease unconjugated MMAE Cmax and AUC exposure with moderate effect, with no change in ADC exposure. The full impact of rifampin on the Cmax of unconjugated MMAE may be underestimated in the PBPK model.
Concomitant use of enfortumab vedotin is predicted not to affect exposure to midazolam (a sensitive CYP3A substrate). In vitro studies using human liver microsomes indicate that unconjugated MMAE inhibits CYP3A4/5 but not other CYP450 isoforms. Unconjugated MMAE did not induce major CYP450 enzymes in human hepatocytes.
In vitro studies
In vitro studies indicate that unconjugated MMAE is a substrate and not an inhibitor of the efflux transporter P-glycoprotein (P-gp). In vitro studies determined that unconjugated MMAE was not a substrate of breast cancer resistance protein (BCRP), multidrug resistance – associated protein 2 (MRP2), organic anion transporting polypeptide 1B1 or 1B3 (OATP1B1 or OATP1B3), organic cation transporter 2 (OCT2), or organic anion transporter 1 or 3 (OAT1 or OAT3). Unconjugated MMAE was not an inhibitor of the bile salt export pump (BSEP), P-gp, BCRP, MRP2, OCT1, OCT2, OAT1, OAT3, OATP1B1, or OATP1B3 at clinically relevant concentrations.
5.3. Preclinical safety data
Genotoxicity studies showed that MMAE had no discernible genotoxic potential in a reverse mutation test in bacteria (Ames test) or in a L5178Y TK+/- mouse lymphoma mutation assay. MMAE did induce chromosomal aberrations in the micronucleus test in rats which is consistent with the pharmacological action of microtubule-disrupting agents.
Skin lesions were noted in repeat dose studies in rats (4- and 13-weeks) and in monkeys (4-weeks). The skin changes were fully reversible by the end of a 6-week recovery period.
Hyperglycaemia reported in the clinical studies was absent in both the rat and monkey toxicity studies and there were no histopathological findings in the pancreas of either species.
Foetal toxicity (reduced litter size or complete litter loss) was observed and decrease in the litter size was reflected in an increase in early resorptions. Mean foetal body weight in the surviving foetuses at the 2 mg/kg dose level were reduced compared with control.
Enfortumab vedotin associated foetal skeletal variations were considered developmental delays. A dose of 2 mg/kg (approximately similar to the exposure at the recommended human dose) resulted in maternal toxicity, embryo-foetal lethality and structural malformations that included gastroschisis, malrotated hindlimb, absent forepaw, malpositioned internal organs and fused cervical arch. Additionally, skeletal anomalies (asymmetric, fused, incompletely ossified, and misshapen sternebrae, misshapen cervical arch, and unilateral ossification of the thoracic centra) and decreased foetal weight were observed.
Testicular toxicity observed, only in rats, was partially reversed by the end of a 24-week recovery period.
No dedicated preclinical safety studies were conducted with enfortumab vedotin in combination with pembrolizumab.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.