PECFENT Nasal spray, solution Ref.[7244] Active ingredients: Fentanyl
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: Kyowa Kirin Holdings B.V., Bloemlaan 2, 2132NP Hoofddorp, The Netherlands
Pharmacodynamic properties
Pharmacotherapeutic group: Analgesics; opioids; phenylpiperidine derivatives
ATC code: N02AB03
Mechanism of action
Fentanyl is an opioid analgesic, interacting predominantly with the opioid μ-receptor. Its primary therapeutic actions are analgesia and sedation. Secondary pharmacological effects are respiratory depression, bradycardia, hypothermia, constipation, miosis, physical dependence and euphoria.
Opioids may influence the hypothalamic-pituitary-adrenal or –gonadal axes. Some changes that can be seen include an increase in serum prolactin, and decreases in plasma cortisol and testosterone. Clinical signs and symptoms may be manifest from these hormonal changes.
Pharmacodynamic effects
A double-blind, randomised, placebo-controlled crossover study has been conducted in which 114 patients who experienced on average 1 to 4 episodes of break through pain (BTP) per day while taking maintenance opioid therapy were entered into an initial open-label titration phase in order to identify an effective dose of PecFent (Study CP043). The patients entering the double-blind phase treated up to 10 episodes of BTP with either PecFent (7 episodes) or placebo (3 episodes) in a random order.
Of the patients entering the titration phase, only 7 (6.1%) were unable to be titrated to an effective dose due to lack of efficacy and 6 (5.3%) withdrew due to adverse events.
The primary endpoint was the comparison between the summed pain intensity difference at 30 minutes after dosing (SPID30), which was 6.57 in the PecFent-treated episodes compared to 4.45 for placebo (p<0.0001). The SPID for PecFent-treated episodes was also significantly different to placebo at 10 15, 45 and 60 minutes after administration.
The mean pain intensity scores (73 patients) for all PecFent-treated episodes (459 episodes) compared to those treated with placebo (200 episodes) were significantly lower at 5, 10, 15, 30, 45 and 60 minutes following administration (see Figure 1).
Figure 1. Mean (± SE) Pain Intensity Scores at Each Time Point (mITT Population):
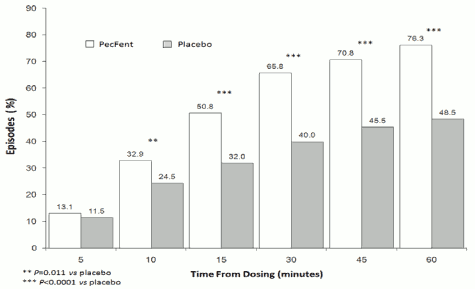
The superior efficacy of PecFent over placebo was supported by data from secondary endpoints including the number of BTP episodes with clinically meaningful pain relief, defined as a reduction in pain intensity score of at least 2 (Figure 2).
Figure 2. Clinically Meaningful Pain Relief – PecFent vs placebo: % Patients' Episodes With ≥2 Point Reduction in Pain Intensity:
In a double-blind, randomized comparator-controlled study (Study 044) of similar design to Study 043 conducted in opioid-tolerant patients with breakthrough cancer pain on stable doses of regularly scheduled opioids, PecFent was shown to be superior to immediate-release morphine sulfate (IRMS). Superiority was demonstrated by the primary endpoint, Pain Intensity Difference within 15 minutes, which was 3.02 in patients treated with PecFent compared to 2.69 in patients treated with IRMS (p=0.0396).
In a long-term, open-label, safety study (Study 045), 355 patients entered the 16-week treatment phase, during which 42,227 episodes of breakthrough cancer pain (BTP) were treated with PecFent. One hundred of these patients continued treatment for up to 26 months in an extension phase. Of the 355 patients treated in the open-label treatment phase, 90% required no increase in dose.
In the randomised, placebo-controlled study (CP043) 9.4% of 459 PecFent-treated BTP episodes in 73 patients required use of any further (rescue) medicinal products within 60 minutes of dosing. During the longer-term, open-label study (CP045) this was 6.0% of 42,227 episodes in 355 patients treated with PecFent during up to 159 days of treatment.
Pharmacokinetic properties
General introduction
Fentanyl is highly lipophilic and can be absorbed very rapidly through the nasal mucosa and more slowly by the gastrointestinal route. It is subject to first pass hepatic and intestinal metabolism and the metabolites do not contribute to fentanyl’s therapeutic effects.
PecFent utilises the PecSys nasal drug delivery system to modulate the delivery and absorption of fentanyl. The PecSys system allows the product to be sprayed into the front area of the nasal cavity as a fine mist of droplets, which gel on contact with the calcium ions present in the nasal mucosa. Fentanyl diffuses from the gel and is absorbed through the nasal mucosa; this gel-modulated absorption of fentanyl restrains the peak in plasma concentration (Cmax) whilst allowing the attainment of an early time to that peak (Tmax).
Absorption
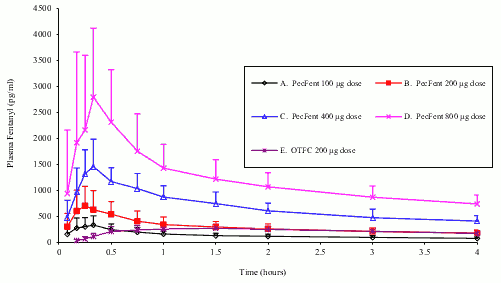
In a pharmacokinetic study comparing PecFent (100, 200, 400 and 800 micrograms) with oral transmucosal fentanyl citrate (OTFC, 200 micrograms), fentanyl was shown to be rapidly absorbed following single dose intranasal administration of PecFent, with median Tmax ranging from 15 to 21 minutes (Tmax for OTFC was approximately 90 minutes). The variability of the pharmacokinetics of fentanyl was considerable following treatment with both PecFent and OTFC. Relative bioavailability of fentanyl from the PecFent treatment compared to the 200 microgram OTFC was approximately 120%.
The main pharmacokinetic parameters are shown in the following table.
Pharmacokinetic parameters in adult subjects receiving PecFent and OTFC:
| Pharmacokinetic parameters (mean (%CV)) | PecFent | OTFC | |||
|---|---|---|---|---|---|
| 100 micrograms | 200 micrograms | 400 micrograms | 800 micrograms | 200 micrograms | |
| Tmax (hours)* | 0.33 (0.08-1.50) | 0.25 (0.17-1.60) | 0.35 (0.25-0.75) | 0.34 (0.17-3.00) | 1.50 (0.50-8.00) |
| Cmax (pg/ml) | 351.5 (51.3) | 780.8 (48.4) | 1552.1 (26.2) | 2844.0 (56.0) | 317.4 (29.9) |
| AUC (pg.hour/ml) | 2460.5 (17.9) | 4359.9 (29.8) 7513 | 4 (26.7) | 17272 (48.9) | 3735.0 (32.8) |
| t1/2 (hour) | 21.9 (13.6) | 24.9 (51.3) | 15.0 (24.7) | 24.9 (92.5) | 18.6 (31.4) |
* Data for Tmax presented as median (range).
The curves for each dose level are similar in shape with increasing dose levels producing increasing plasma fentanyl levels. Dose-proportionality was demonstrated for Cmax and area under the curve (AUC) in the dose range 100 micrograms to 800 micrograms (see Figure 3). If switching to PecFent from another fentanyl product for BTP, independent dose titration with PecFent is required as the bioavailability between products differs significantly.
Figure 3. Mean plasma fentanyl concentrations following single doses of PecFent and OTFC in healthy subjects:
A pharmacokinetic study was conducted to evaluate the absorption and tolerability of a single dose of PecFent in patients with pollen-induced seasonal allergic rhinitis, comparing the un-challenged, acutely challenged (rhinitic) and acutely challenged and then treated with oxymetazoline, states.
There was no clinically significant effect of acute rhinitis on Cmax, Tmax or overall exposure to fentanyl, comparing the unchallenged with the acutely challenged states. Following treatment of the acute rhinitic state with oxymetazoline, there were reductions in Cmax and exposure, and increases in Tmax that were statistically, and possibly clinically, significant.
Distribution
Fentanyl is highly lipophilic and is well distributed beyond the vascular system, with a large apparent volume of distribution. Animal data have shown that, following absorption, fentanyl is rapidly distributed to the brain, heart, lungs, kidneys and spleen followed by a slower redistribution to muscles and fat.
The plasma protein binding of fentanyl is 80–85%. The main binding protein is alpha-1-acid glycoprotein, but both albumin and lipoproteins contribute to some extent. The free fraction of fentanyl increases with acidosis.
Biotransformation
The metabolic pathways following nasal administration of PecFent have not been characterised in clinical studies. Fentanyl is metabolised in the liver to norfentanyl by cytochrome CYP3A4 isoform. Norfentanyl is not pharmacologically active in animal studies. It is more than 90% eliminated by biotransformation to N-dealkylated and hydroxylated inactive metabolites.
Elimination
Disposition of fentanyl following intranasal administration of PecFent has not been characterised in a mass balance study. Less than 7% of an administered dose of fentanyl is excreted unchanged in the urine and only about 1% is excreted unchanged in the faeces. The metabolites are mainly excreted in the urine, while faecal excretion is less important.
The total plasma clearance of fentanyl following intravenous administration is approximately 42 L/h.
Linearity/non-linearity
Dose-proportionality was demonstrated for Cmax and AUC in the dose range 100 micrograms to 800 micrograms.
The effect of renal or hepatic impairment on the pharmacokinetics of PecFent has not been studied.
Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity and carcinogenicity.
Embryo-foetal developmental toxicity studies conducted in rats and rabbits revealed no compound-induced malformations or developmental variations when administered during the period of organogenesis.
In a fertility and early embryonic development study in rats, a male-mediated effect was observed at high doses (300 mcg/kg/day, s.c.) and is consistent with the sedative effects of fentanyl in animal studies.
In studies on pre and postnatal development in rats the survival rate of offspring was significantly reduced at doses causing severe maternal toxicity. Further findings at maternally toxic doses in F1 pups were delayed physical development, sensory functions, reflexes and behaviour. These effects could either be indirect effects due to altered maternal care and/or decreased lactation rate or a direct effect of fentanyl on the pups.
Carcinogenicity studies (26-week dermal alternative bioassay in Tg.AC transgenic mice; two-year subcutaneous carcinogenicity study in rats) with fentanyl did not induce any findings indicative of oncogenic potential. Evaluation of brain slides from the carciogenicity study in rats revealed brain lesions in animals administered high doses of fentanyl citrate. The relevance of these findings to humans is unknown.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.