Portrazza Solution for injection Ref.[11133] Active ingredients: Human normal immunoglobulin G
Source: FDA, National Drug Code (US) Revision Year: 2017
12.1. Mechanism of Action
Necitumumab is a recombinant human lgG1 monoclonal antibody that binds to the human epidermal growth factor receptor (EGFR) and blocks the binding of EGFR to its ligands. Expression and activation of EGFR has been correlated with malignant progression, induction of angiogenesis, and inhibition of apoptosis. Binding of necitumumab induces EGFR internalization and degradation in vitro. In vitro, binding of necitumumab also led to antibody-dependent cellular cytotoxicity (ADCC) in EGFR-expressing cells.
In in vivo studies using xenograft models of human cancer, including non-small cell lung carcinoma, administration of necitumumab to implanted mice resulted in increased antitumor activity in combination with gemcitabine and cisplatin as compared to mice receiving gemcitabine and cisplatin alone.
12.3. Pharmacokinetics
Based on population pharmacokinetic (popPK) analysis of serum concentration data from patients in clinical studies with PORTRAZZA, necitumumab exhibits dose-dependent kinetics. Following the administration of PORTRAZZA 800 mg on Days 1 and 8 of each 21 day cycle, the estimated mean total systemic clearance (CLtot) at steady state is 14.1 mL/h (CV=39%), the steady state volume of distribution (Vss) is 7.0 L (CV=31%) and the elimination half-life is approximately 14 days. The predicted time to reach steady state is approximately 100 days.
Specific Populations
Effect of Age, Body Weight, Sex and Race
Based on the popPK analysis with data obtained in 807 patients, age (range 19-84 years), sex (75% males), and race (85% Whites) have no effect on the systemic exposure of necitumumab.
Body weight is identified as a covariate in the popPK analysis; however, weight-based dosing is not expected to significantly decrease the variability in exposure. No dose adjustment based on body weight is necessary.
Renal Impairment
Patients with Renal Impairment — PopPK analysis did not identify a correlation between necitumumab exposure and renal function as assessed by estimated creatinine clearance ranging from 11-250 mL/min.
Hepatic Impairment
Patients with Hepatic Impairment — PopPK analysis did not identify a correlation between the exposure of necitumumab and hepatic function as assessed by alanine aminotransferase (ranging from 2-615 U/L), aspartate transaminase (ranging from 1.2-619 U/L) and total bilirubin (ranging from 0.1-106 μmol/L).
Drug Interactions
Effect of Necitumumab on Gemcitabine and Cisplatin
In 12 patients with advanced solid tumors who received gemcitabine and cisplatin in combination with PORTRAZZA, the geometric mean dose-normalized AUC of gemcitabine was increased by 22% and Cmax increased by 63% compared to administration of gemcitabine and cisplatin alone while exposure to cisplatin was unchanged.
Effect of Gemcitabine and Cisplatin on Necitumumab
Concomitant administration of gemcitabine and cisplatin had no effect on the exposure of necitumumab.
Immunogenicity
In Study 1, the CLtot of necitumumab was 26% higher and Css,ave was 34% lower in patients who tested positive for anti-necitumumab antibodies (ADA) post-treatment than patients who tested negative for ADA post-treatment.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been performed to assess the potential of necitumumab for carcinogenicity or genotoxicity.
Fertility studies have not been performed with necitumumab.
14. Clinical Studies
14.1 Squamous Non-Small Cell Lung Cancer
Study 1 was a randomized, multi-center open-label, controlled trial conducted in 1093 patients receiving gemcitabine and cisplatin first-line chemotherapy for metastatic squamous NSCLC. Patients were randomized (1:1) to receive PORTRAZZA plus gemcitabine and cisplatin or gemcitabine and cisplatin alone. Stratification factors were ECOG performance status (0, 1 versus 2) and geographic region (North America, Europe, and Australia versus South America, South Africa, and India versus Eastern Asia). Gemcitabine (1250 mg/m², Days 1 and 8) plus cisplatin (75 mg/m², Day 1) were administered every 3 weeks (1 cycle) for a maximum of 6 cycles in the absence of disease progression or unacceptable toxicity. PORTRAZZA (800 mg by intravenous infusion on Days 1 and 8 of each 3-week cycle) was administered prior to gemcitabine and cisplatin. Patients demonstrating at least stable disease on PORTRAZZA plus gemcitabine and cisplatin were to continue PORTRAZZA as a single agent in the absence of disease progression or unacceptable toxicity after completion of 6 planned courses of chemotherapy or if chemotherapy was discontinued for toxicity.
Of the 1093 randomized patients, the median age was 62 years (range 32 to 86), 83% were male; 84% were Caucasian; and 91% were smokers. The majority of the patients (87%) were enrolled in North America, Europe and Australia, 36 patients (3%) were enrolled at clinical sites in the U.S., 6% of the patients were enrolled in South America, South Africa and India and 8% enrolled at clinical sites in Eastern Asia. Baseline ECOG performance status was 0 or 1 for 91%, and 2 for 9% of patients; 91% had metastatic disease in 2 or more sites. In the PORTRAZZA plus gemcitabine and cisplatin arm, 51% of patients continued PORTRAZZA after completion or discontinuation of chemotherapy. Use of post-study systemic therapy was 47% in the PORTRAZZA plus gemcitabine and cisplatin arm, and 45% in the gemcitabine and cisplatin arm.
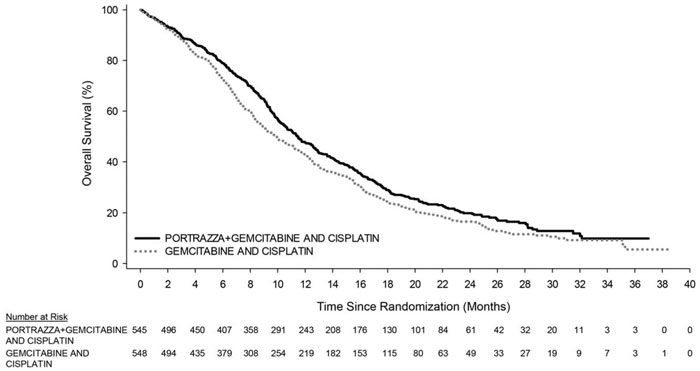
The main outcome measure was overall survival (OS). Investigator-assessed progression-free survival (PFS) and overall response rate (ORR) were also assessed. Overall survival and PFS were statistically significantly improved in patients randomized to receive PORTRAZZA plus gemcitabine and cisplatin compared to gemcitabine and cisplatin alone. There was no difference in ORR between arms, with an ORR of 31% (95% CI 27, 35) for PORTRAZZA plus gemcitabine and cisplatin arm and an ORR of 29% (95% CI 25, 33) for gemcitabine and cisplatin arm, p-value 0.40.
Efficacy results are shown in Table 3 and Figure 1.
Table 3. Efficacy Results for Metastatic Squamous Non-Small Cell Lung Cancer:
| PORTRAZZA PLUS GEMCITABINE AND CISPLATIN N=545 | GEMCITABINE AND CISPLATIN N=548 | |
|---|---|---|
| Overall Survival | ||
| Number of deaths (%) | 418 (77%) | 442 (81%) |
| Median – months (95% CI)a | 11.5 (10.4, 12.6) | 9.9 (8.9, 11.1) |
| Stratified Hazard Ratio (95% CI) | 0.84 (0.74, 0.96) | |
| Stratified Log-rank p-value | 0.01 | |
| Progression-Free Survivalb | ||
| Number of events (%) | 431 (79%) | 417 (76%) |
| Median – months (95% CI) | 5.7 (5.6, 6.0) | 5.5 (4.8, 5.6) |
| Stratified Hazard Ratio (95% CI) | 0.85 (0.74, 0.98) | |
| Stratified Log-rank p-value | 0.02 | |
a Abbreviations: CI = confidence interval
b Investigator assessed
Figure 1. Kaplan-Meier Curves of Overall Survival in Patients with Metastatic Squamous Non-Small Cell Lung Cancer:
14.2 Non-Squamous NSCLC – Lack of Efficacy
Lack of efficacy of PORTRAZZA in combination with pemetrexed and cisplatin for the treatment of patients with metastatic non-squamous non-small cell lung cancer was determined in one randomized, open-label, multicenter trial (Study 2). The study was closed prematurely after 633 patients were enrolled due to increased incidence of death due to any cause and of thromboembolic events in the PORTRAZZA arm. Patients with no prior chemotherapy for metastatic disease were randomized (1:1) to receive PORTRAZZA plus pemetrexed and cisplatin or pemetrexed and cisplatin alone. Stratification factors were smoking status (non-smokers versus light smokers versus smokers), ECOG performance status (0-1 versus 2), histology (adenocarcinoma/large cell versus others), and geographic region. PORTRAZZA (800 mg, Days 1 and 8 of each 3-week cycle) was administered prior to pemetrexed and cisplatin. Patients demonstrating at least stable disease on PORTRAZZA plus pemetrexed and cisplatin were to continue PORTRAZZA as a single agent in the absence of disease progression or unacceptable toxicity after completion of 6 planned courses of chemotherapy.
Of the 633 patients, 315 were randomized to PORTRAZZA plus pemetrexed and cisplatin arm and 318 in the pemetrexed and cisplatin arm. The median age was 61 years, 67% were male, 93% were Caucasian and 94% had ECOG PS 0 or 1. More than 75% were smokers and 89% had adenocarcinoma histology.
The main efficacy outcome was OS. Progression-free survival and ORR were also assessed. Addition of PORTRAZZA to pemetrexed and cisplatin did not improve OS [HR=1.01; 95%CI (0.84, 1.21); p-value = 0.96)]; PFS [HR=0.96; 95% CI (0.8, 1.16)] or ORR (31% in the PORTRAZZA plus pemetrexed and cisplatin arm and 32% in the pemetrexed and cisplatin alone arm).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.