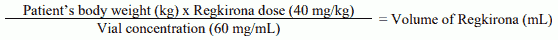REGKIRONA Concentrate for solution for infusion Ref.[27956] Active ingredients: Regdanvimab
Revision Year: 2021 Publisher: Celltrion Healthcare Hungary Kft., 1062 Budapest, Váci út 1-3. WestEnd Office Building B torony, Hungary
4.1. Therapeutic indications
Regdanvimab is indicated for the treatment of adults with coronavirus disease 2019 (COVID-19) who do not require supplemental oxygen and who are at increased risk of progressing to severe COVID-19 (see section 5.1).
4.2. Posology and method of administration
Regdanvimab should only be administered in settings in which health care providers have immediate access to appropriate resuscitation equipment and medicinal products to treat a severe infusion reaction, including anaphylaxis, and where patients can be clinically monitored during administration and be observed for at least 1 hour after infusion is complete (see section 4.4).
Posology
The recommended dosage of regdanvimab in adults is a single IV infusion of 40 mg/kg. Regdanvimab should be administered within 7 days of onset of symptoms of COVID-19 (see section 5.1).
The volume of Regkirona is calculated as follows.
Calculation to determine the total volume of Regkirona to be administered:
Calculation to determine the total number of Regkirona vials needed:
Table 1. Sample calculations for patients receiving the recommended dose of 40 mg/kg of Regkirona for weights ranging from 40 kg to 120 kg:
| Body weight (kg) | Total dose (mg) | Volume (mL) | Vials (n) |
|---|---|---|---|
| 40 | 1,600 | 27 | 2 |
| 60 | 2,400 | 40 | 3 |
| 80 | 3,200 | 53 | 4 |
| 100 | 4,000 | 67 | 5 |
| 120 | 4,800 | 80 | 5 |
Note: If a patient’s weight is more than 200 kg, the dose calculation should use 200 kg. The maximal recommended dose is 8,000 mg.
Special populations
Elderly
No dose adjustment of regdanvimab is required in elderly patients (see section 5.2).
Renal impairment
No dose adjustments are recommended.
Hepatic impairment
No dose adjustments are recommended.
Paediatric population
The safety and efficacy of regdanvimab in paediatric patients have not yet been established. No data are available.
Method of administration
For intravenous use only.
Regdanvimab should be diluted and administered intravenously over 60 minutes.
The rate of infusion may be slowed or interrupted if the patient develops any signs of infusion-related reactions or other adverse reactions and appropriate treatment should be initiated as necessary (see section 4.4).
For instructions on dilution of the medicinal product before administration, see section 6.6.
4.9. Overdose
Single doses up to 8,000 mg have been administered in clinical trials without dose-limiting toxicity. Treatment of overdose should consist of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient. There is no specific antidote for overdose with regdanvimab.
6.3. Shelf life
Unopened vials: One year.
Diluted solution for infusion: Chemically and physical in-use stability has been demonstrated for 72 hours at 2°C-8°C or 4 hours at ≤30°C after dilution in sodium chloride 9 mg/mL (0.9%) solution for infusion.
From a microbiological point of view, the prepared infusion solution should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2°C-8°C, unless dilution has taken place in controlled and validated aseptic conditions.
6.4. Special precautions for storage
Store in a refrigerator (2°C-8°C).
Do not freeze. Keep the medicinal product in its outer carton in order to protect from light.
For storage conditions after dilution of the medicinal product, see section 6.3.
6.5. Nature and contents of container
Type I glass vial with a chlorobutyl rubber stopper.
Pack size of 1 vial.
6.6. Special precautions for disposal and other handling
Preparation
Regkirona solution for infusion should be prepared by a qualified healthcare professional using aseptic technique:
- Remove Regkirona vial(s) from refrigerated storage and allow to equilibrate to room temperature (not exceeding 30°C) for approximately 20 minutes before preparation. Do not expose to direct heat. Do not shake the vial(s).
- Regkirona is a clear to opalescent, colourless to pale yellow solution for infusion. Inspect Regkirona vial(s) visually for particulate matter and discolouration prior to dilution. Should either be observed, the vial(s) must be discarded, and new vial(s) should be used for preparation.
- Calculate total volume of Regkirona to be administered (see section 4.2).
- Dilute Regkirona in a bag containing sodium chloride 9 mg/mL (0.9%) solution for infusion. The total volume of the medicinal product and sodium chloride should be 250 mL.
- In a 250 mL bag of sodium chloride, withdraw and discard the required volume (which is identical to the calculated volume of Regkirona) of sodium chloride 9 mg/mL (0.9%) from the infusion bag.
- Withdraw the calculated volume of Regkirona from the vial(s) using a sterile syringe.
- Transfer Regkirona to the infusion bag.
- Gently invert IV bag by hand approximately 10 times to mix. Do not shake.
Administration
Regkirona solution for infusion should be administered by a qualified healthcare professional.
- Gather the recommended materials for infusion: Infusion set with in-line filter (PES (Polyethersulfone) filter with a pore size of 1.2 μm or less would be recommended).
- Attach the infusion set to the IV bag.
- Prime the infusion set.
- Administer as an IV infusion via pump over 60 minutes.
- The prepared solution for infusion should not be administered simultaneously with any other medicinal product.
Disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.