REZENOPY Nasal spray Ref.[109668] Active ingredients: Naloxone
Source: FDA, National Drug Code (US) Revision Year: 2024
12.1. Mechanism of Action
Naloxone hydrochloride is an opioid antagonist that antagonizes opioid effects by competing for the same receptor sites.
Naloxone hydrochloride reverses the effects of opioids, including respiratory depression, sedation, and hypotension. It can also reverse the psychotomimetic and dysphoric effects of agonist-antagonists such as pentazocine.
12.2. Pharmacodynamics
When naloxone hydrochloride is administered intravenously, the onset of action is generally apparent within two minutes. The time to onset of action is shorter for intravenous compared to subcutaneous or intramuscular routes of administration. The duration of action is dependent upon the dose and route of administration of naloxone hydrochloride.
12.3. Pharmacokinetics
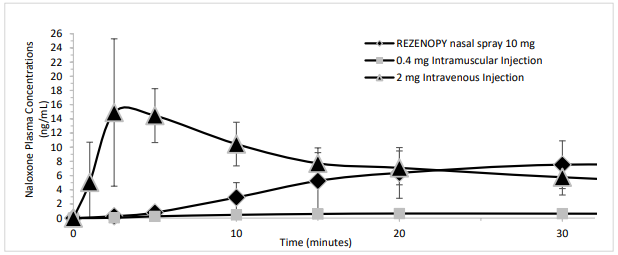
In a pharmacokinetic study in 30 healthy adult subjects, the relative bioavailability (BA) of one nasal spray of a 10 mg total dose (0.11 mL of 91 mg/mL naloxone hydrochloride solution) was compared to a single dose of 0.4 mg naloxone hydrochloride intramuscular injection and a single dose of 2 mg naloxone hydrochloride intravenous injection. For intranasal administration, the subjects were instructed not to breathe through the nose during administration of the nasal spray and remained fully supine for approximately one-hour post-dose. For intramuscular administration, naloxone was administered as a single injection in the gluteus maximus muscle. For intravenous administration, naloxone was administered as an intravenous bolus. The pharmacokinetic parameters obtained in the study are shown in Table 1.
Table 1. Mean Pharmacokinetic Parameters (CV%) for REZENOPY nasal spray, Intramuscular and Intravenous Injections of Naloxone HCl to Healthy Subjects:
| Parameter | REZENOPY nasal spray 10 mg (n=29) | Naloxone HCl 0.4 mg Intramuscular Injection (n=30) | Naloxone HCl 2 mg Intravenous Injection (n=23) |
|---|---|---|---|
| tmax (h)† | 0.75 (0.25, 1.03) | 0.50 (0.17, 2.00) | 0.08 (0.02, 0.18) |
| Cmax (ng/mL) | 9.11 (35.45) | 0.74 (36.63) | 18.41 (46.08) |
| AUCt(h·ng/mL) | 19.19 (24.81) | 1.92 (19.75) | 12.18 (24.30) |
| AUC0-∞(h·ng/mL) | 19.52 (24.78) | 1.98 (19.18) | 12.25 (24.22) |
| t½ (h) | 1.33 (16.09) | 1.22 (18.48) | 1.18 (11.59) |
| Dose normalized absolute BA vs. IV†† | 0.34 (30.53) | 0.84 (29.06) | - |
† tmax reported as median (minimum, maximum)
†† N=22 and N=23, respectively for REZENOPY nasal spray 10 mg and Naloxone HCl 0.4 mg Intramuscular Injection for Dose normalized absolute BA vs. IV
Figure 1. Mean ± SD Plasma Concentration of Naloxone 0-30 minutes Following Intranasal, Intramuscular and Intravenous Administration:
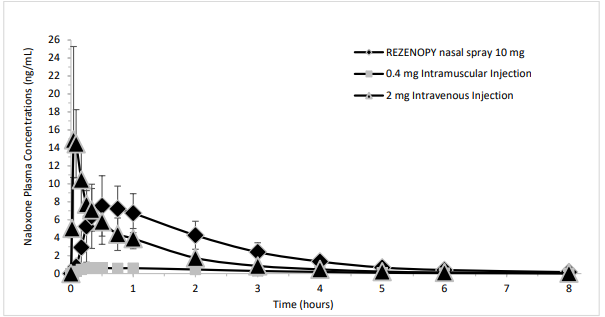
Figure 2. Mean ± SD Plasma Concentration of Naloxone 0-8 hours Following Intranasal, Intramuscular and Intravenous Administration:
Absorption
REZENOPY nasal spray showed median Tmax 0.75 hour. The median Tmax for the 0.4 mg naloxone hydrochloride intramuscular injection was 0.5 hour. The dose normalized absolute bioavailability of one dose (10 mg) of REZENOPY nasal spray and 0.4 mg of naloxone HCl intramuscular injection compared to 2 mg of naloxone HCl intravenous injection was 34% and 84% respectively.
Distribution
Following parenteral administration, naloxone is distributed in the body and readily crosses the placenta. Plasma protein binding occurs but is relatively weak. Plasma albumin is the major binding constituent, but significant binding of naloxone also occurs to plasma constituents other than albumin. It is not known whether naloxone is excreted into human milk.
Elimination
Following a single intranasal administration of REZENOPY nasal spray (10 mg dose of naloxone hydrochloride), the mean plasma half-life of naloxone in healthy adults was approximately 1.33 hours (16.09% CV) hours. Following the administrations of a 0.4 mg naloxone hydrochloride intramuscular injection and a 2 mg naloxone hydrochloride intravenous injection, the half-life was 1.22 hours (18.48% CV) and 1.18 hours (11.59% CV), respectively.
In a neonatal study of naloxone hydrochloride injection, the mean (± SD) plasma half-life was observed to be 3.1 (± 0.5) hours.
Metabolism
Naloxone hydrochloride is metabolized in the liver, primarily by glucuronide conjugation, with naloxone-3-glucoronide as the major metabolite.
Excretion
After an oral or intravenous dose, about 25-40% of naloxone is excreted as metabolites in urine within 6 hours, about 50% in 24 hours, and 60-70% in 72 hours.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies to evaluate the carcinogenic potential of naloxone have not been completed.
Mutagenesis
Naloxone was weakly positive in the Ames mutagenicity and in the in vitro human lymphocyte chromosome aberration test but was negative in the in vitro Chinese hamster V79 cell HGPRT mutagenicity assay and in the in vivo rat bone marrow chromosome aberration study.
Impairment of Fertility
Reproductive studies conducted in mice and rats at doses 2-times and 4-times, respectively, a human dose of 20 mg/day (from two nasal sprays of REZENOPY) based on body surface area comparison, demonstrated no adverse effects on fertility of naloxone hydrochloride.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.