RYTELO Solution for injection Ref.[110353] Active ingredients: Imetelstat
Source: FDA, National Drug Code (US) Revision Year: 2024
Product description
RYTELO for injection contains imetelstat, an oligonucleotide telomerase inhibitor for intravenous use. Imetelstat sodium is a white to off-white or slightly yellow, amorphous, solid powder. It is highly soluble in aqueous solutions, including in 0.9% Sodium Chloride Injection, at both refrigerated and room temperatures. Imetelstat sodium is hygroscopic.
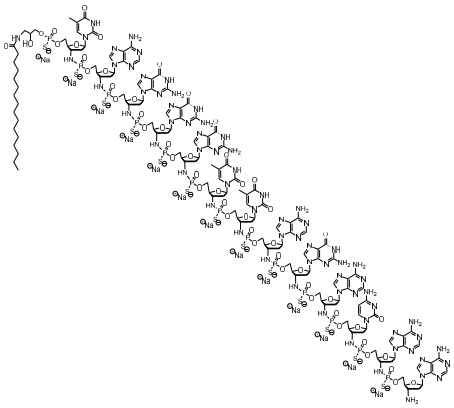
The chemical name for the imetelstat sodium drug substance is DNA, d(3'-amino-3'-deoxy-P-thio) (T-AG-G-G-T-T-A-G-A-C-A-A), 5'[O[2-hydroxy-3-(hexadecanoylamino)propyl] phosphorothioate], sodium salt (1:13). The molecular formula is C148H198N68O53P13S13Na13 (as sodium salt), which equates to a formula weight of 4896 g/mol. The molecular formula for the free acid form is C148H211N68O53P13S13 which equates to a formula weight of 4610 g/mol. The structural formula for imetelstat sodium is:
RYTELO (imetelstat) for injection is a sterile, preservative-free, white to off-white or slightly yellow lyophilized powder for intravenous infusion after reconstitution and dilution. Each single-dose vial provides either 47 mg of imetelstat (equivalent to 50 mg imetelstat sodium) or 188 mg of imetelstat (equivalent to 200 mg imetelstat sodium). The following inactive ingredients may be added during manufacturing: sodium carbonate anhydrous (for the 47 mg preparation)/sodium carbonate monohydrate (for the 188 mg preparation) or hydrochloric acid (to adjust to pH of 7.0 to 8.5).
| Dosage Forms and Strengths |
|---|
|
| How Supplied | ||||||
|---|---|---|---|---|---|---|
|
RYTELO (imetelstat) for injection is a preservative free, white to off-white or slightly yellow, lyophilized powder available as:
Manufactured for: Geron Corporation, 919 E. Hillsdale Blvd., Suite 250, Foster City, CA 94404 Manufactured by (188 mg vials): Catalent Indiana, LLC, 1300 S Patterson Drive, Bloomington, IN 47403 Manufactured by (47 mg vials): Patheon Italia, S.p.A, 2° Trav. SX Via Morolense, 5, 03013 Ferentino (FR), Italy |
Drugs
| Drug | Countries | |
|---|---|---|
| RYTELO | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.