RYZNEUTA Solution for injection Ref.[110038] Active ingredients: Efbemalenograstim alfa
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Evive Biotechnology Ireland LTD, 20 Kildare Street, Dublin 2, D02 T3V7, Ireland
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: immunostimulants, colony stimulating factors
ATC Code: L03AA18
Mechanism of action
Human granulocyte-colony stimulating factor (G-CSF) is a glycoprotein, which regulates the production and release of neutrophils from the bone marrow.
Pharmacodynamic effects
Efbemalenograstim alfa is a recombinant fusion protein containing G-CSF, a 16 amino-acid linker, and the Fc portion of human IgG2. In solution, efbemalenograstim alfa forms covalentlylinked dimers (disulfide bonds between Fc moieties) and has an immunoglobulin-like structure. Efbemalenograstim alfa is a sustained duration form of G-CSF due to decreased renal clearance. Efbemalenograstim alfa and other G-CSFs have identical modes of action, causing a marked increase in peripheral blood neutrophil counts within 24 hours, with minor increases in monocytes and/or lymphocytes.
Neutrophils produced in response to G-CSF show normal or enhanced function as demonstrated by tests of chemotactic and phagocytic function. As with other haematopoietic growth factors, G-CSF has shown in vitro stimulating properties on human endothelial cells. G-CSF can promote growth of myeloid cells, including malignant cells, in vitro and similar effects may be seen on some non-myeloid cells in vitro.
Clinical efficacy and safety
In a randomised, placebo-controlled, double-blind study in patients with breast cancer, the effect of efbemalenograstim alfa on the duration of neutropenia and the incidence of febrile neutropenia was evaluated following administration of a chemotherapy regimen associated with a febrile neutropenia rate of 30-40% (docetaxel 75 mg/m² and doxorubicin 60 mg/m² every 3 weeks for 4 cycles). One hundred twenty-two patients were randomised 2:1 to receive either a single 20 mg dose of efbemalenograstim alfa or placebo approximately 24 hours (day 2) after chemotherapy in cycle 1; all subjects received efbemalenograstim alfa in cycles 2–4. The primary endpoint of mean duration of grade 4 neutropenia in cycle 1 was lower for patients randomised to receive efbemalenograstim alfa compared with placebo (1.3 days versus 3.9 days, p<0.001), as was the incidence of febrile neutropenia (5% versus 26%, p<0.001). Consistent with the reduction in febrile neutropenia, the incidence of IV anti-infective use in cycle 1 was also lower in the efbemalenograstim alfa group compared with placebo (4% versus 18%).
Two additional randomised, active-controlled studies compared efbemalenograstim alfa, given as a once-per-cycle 20 mg dose, to either once-per-cycle pegfilgrastim (n=393) or daily filgrastim (n=239) for reducing the duration of neutropenia and the incidence of febrile neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. In the pegfilgrastim comparison, patients with metastatic or non-metastatic breast cancer received a docetaxel and cyclophosphamide regimen. In this study, the mean duration of grade 4 neutropenia in cycle 1 for both the efbemalenograstim alfa and pegfilgrastim groups was 0.2 days (difference 0.0 days, 95% CI -0.1, 0.1). Over the entire study, the rate of febrile neutropenia was 3.0% of efbemalenograstim alfa-treated patients compared with 0.5% of pegfilgrastim-treated patients (difference 2.5%, 95% CI -7.3%, 12.4%). In the comparison to filgrastim (median of 8 daily doses), patients with non-metastatic breast cancer received an epirubicin and cyclophosphamide regimen. In this study, the mean duration of grade 4 neutropenia in cycle 1 for the efbemalenograstim alfa group was 0.3 days and in the filgrastim group was 0.2 days (median difference 0.0 days, 95% CI -0.0, 0.0). Over the entire study, the rate of febrile neutropenia was 0.8% of efbemalenograstim alfa-treated patients compared with 1.7% of filgrastim-treated patients (difference -0.8%, 95% CI -4%, 2%).
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with Ryzneuta in one or more subsets of the paediatric population in the treatment of chemotherapy-induced neutropenia (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Absorption
After subcutaneous injection of efbemalenograstim alfa, the peak serum concentration of efbemalenograstim alfa occurs at 36 hours [min-max: 6-96 hours] after dosing and serum concentrations of efbemalenograstim alfa are maintained during the period of neutropenia after myelosuppressive chemotherapy.
Distribution
The apparent volume of distribution ranges from 395 to 5679 mL/kg.
Biotransformation
Efbemalenograstim alfa is expected to be metabolized into small peptides by catabolic pathways.
Elimination
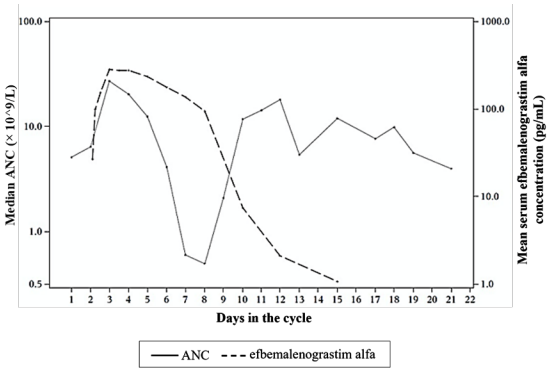
Efbemalenograstim alfa appears to be mainly eliminated by neutrophil-mediated clearance, which becomes saturated at higher doses. Consistent with a self-regulating clearance mechanism, the serum concentration of efbemalenograstim alfa declines rapidly at the onset of neutrophil recovery (see Figure 1). The half-life ranged from 19 to 84 hours after subcutaneous injection.
Linearity/non-linearity
Efbemalenograstim alfa exhibited non-linearity and time-dependent pharmacokinetics over the dose range of 30 to 360 mcg/kg.
Figure 1. Profile of median efbemalenograstim alfa serum concentration and Absolute Neutrophil Count (ANC) in chemotherapy treated patients after a single 320 mcg/kg injection:
Due to the neutrophil-mediated clearance mechanism, the pharmacokinetics of efbemalenograstim alfa is not expected to be affected by renal or hepatic impairment (see section 4.2).
Elderly
Limited data indicate that the pharmacokinetics of efbemalenograstim alfa in elderly subjects (>65 years) is similar to that in adults.
Paediatric population
There are no data available on the pharmacokinetics of efbemalenograstim alfa in children.
5.3. Preclinical safety data
Preclinical data from conventional studies of repeated dose toxicity revealed the expected pharmacological effects including increases in leukocyte count, myeloid hyperplasia in bone marrow, extramedullary haematopoiesis and splenic enlargement.
There were no adverse events observed in offspring from pregnant rats or rabbits given efbemalenograstim alfa subcutaneously at cumulative doses approximately 2.6 and 0.7 times, respectively, the recommended human dose. Similar studies of other G-CSF products in rabbits have shown embryo/foetal toxicity (embryo loss) at cumulative doses approximately 4 times the recommended human dose, which were not seen when pregnant rabbits were exposed to the recommended human dose. Studies in rats indicated that reproductive performance, fertility, oestrous cycling, days between pairing and coitus, and intrauterine survival were unaffected by efbemalenograstim alfa given subcutaneously. The relevance of these findings for humans is not known.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.