SEROQUEL Film-coated tablet Ref.[50528] Active ingredients: Quetiapine
Source: FDA, National Drug Code (US) Revision Year: 2022
12.1. Mechanism of Action
The mechanism of action of quetiapine in the listed indications is unclear. However, the efficacy of quetiapine in these indications could be mediated through a combination of dopamine type 2 (D2) and serotonin type 2 (5HT2) antagonism. The active metabolite, N-desalkyl quetiapine (norquetiapine), has similar activity at D2, but greater activity at 5HT2A receptors, than the parent drug (quetiapine).
12.2. Pharmacodynamics
Quetiapine and its metabolite, norquetiapine, have affinity for multiple neurotransmitter receptors with norquetiapine binding with higher affinity than quetiapine in general. The Ki values for quetiapine and norquetiapine at the dopamine D1 are 428/99.8 nM, at D2 626/489nM, at serotonin 5HT1A 1040/191 nM at 5HT2A 38/2.9 nM, at histamine H1 4.4/1.1 nM, at muscarinic M1 1086/38.3 nM, and at adrenergic α1b 14.6/46.4 nM and, at α2 receptors 617/1290 nM, respectively.
Quetiapine and norquetiapine lack appreciable affinity to the benzodiazepine receptors.
Effect on QT Interval
In clinical trials, quetiapine was not associated with a persistent increase in QT intervals. However, the QT effect was not systematically evaluated in a thorough QT study. In post marketing experience, there were cases reported of QT prolongation in patients who overdosed on quetiapine [see Overdosage (10.1)], in patients with concomitant illness, and in patients taking medicines known to cause electrolyte imbalance or increase QT interval.
12.3. Pharmacokinetics
Adults
Quetiapine activity is primarily due to the parent drug. The multiple-dose pharmacokinetics of quetiapine are dose-proportional within the proposed clinical dose range, and quetiapine accumulation is predictable upon multiple dosing. Elimination of quetiapine is mainly via hepatic metabolism with a mean terminal half-life of about 6 hours within the proposed clinical dose range. Steady-state concentrations are expected to be achieved within two days of dosing. Quetiapine is unlikely to interfere with the metabolism of drugs metabolized by cytochrome P450 enzymes.
Children and Adolescents
At steady state the pharmacokinetics of the parent compound, in children and adolescents (10-17 years of age), were similar to adults. However, when adjusted for dose and weight, AUC and Cmax of the parent compound were 41% and 39% lower, respectively, in children and adolescents than in adults. For the active metabolite, norquetiapine, AUC and Cmax were 45% and 31% higher, respectively, in children and adolescents than in adults. When adjusted for dose and weight, the pharmacokinetics of the metabolite, norquetiapine, was similar between children and adolescents and adults [see Use in Specific Populations (8.4)].
Absorption
Quetiapine is rapidly absorbed after oral administration, reaching peak plasma concentrations in 1.5 hours. The tablet formulation is 100% bioavailable relative to solution. The bioavailability of quetiapine is marginally affected by administration with food, with Cmax and AUC values increased by 25% and 15%, respectively.
Distribution
Quetiapine is widely distributed throughout the body with an apparent volume of distribution of 10±4 L/kg. It is 83% bound to plasma proteins at therapeutic concentrations. In vitro, quetiapine did not affect the binding of warfarin or diazepam to human serum albumin. In turn, neither warfarin nor diazepam altered the binding of quetiapine.
Metabolism and Elimination
Following a single oral dose of 14C-quetiapine, less than 1% of the administered dose was excreted as unchanged drug, indicating that quetiapine is highly metabolized. Approximately 73% and 20% of the dose was recovered in the urine and feces, respectively.
Quetiapine is extensively metabolized by the liver. The major metabolic pathways are sulfoxidation to the sulfoxide metabolite and oxidation to the parent acid metabolite; both metabolites are pharmacologically inactive. In vitro studies using human liver microsomes revealed that the cytochrome P450 3A4 isoenzyme is involved in the metabolism of quetiapine to its major, but inactive, sulfoxide metabolite and in the metabolism of its active metabolite N-desalkyl quetiapine.
Age
Oral clearance of quetiapine was reduced by 40% in elderly patients (≥ 65 years, n=9) compared to young patients (n=12), and dosing adjustment may be necessary [see Dosage and Administration (2.3)].
Gender
There is no gender effect on the pharmacokinetics of quetiapine.
Race
There is no race effect on the pharmacokinetics of quetiapine.
Smoking
Smoking has no effect on the oral clearance of quetiapine.
Renal Insufficiency
Patients with severe renal impairment (Clcr=10-30 mL/min/1.73 m², n=8) had a 25% lower mean oral clearance than normal subjects (Clcr >80 mL/min/1.73 m², n=8), but plasma quetiapine concentrations in the subjects with renal insufficiency were within the range of concentrations seen in normal subjects receiving the same dose. Dosage adjustment is therefore not needed in these patients [see Use in Specific Populations (8.6)].
Hepatic Insufficiency
Hepatically impaired patients (n=8) had a 30% lower mean oral clearance of quetiapine than normal subjects. In two of the 8 hepatically impaired patients, AUC and Cmax were 3 times higher than those observed typically in healthy subjects. Since quetiapine is extensively metabolized by the liver, higher plasma levels are expected in the hepatically impaired population, and dosage adjustment may be needed [see Dosage and Administration (2.4) and Use in Specific Populations (8.7)].
Drug-Drug Interaction Studies
The in vivo assessments of effect of other drugs on the pharmacokinetics of quetiapine are summarized in Table 17 [see Dosage and Administration (2.5 and 2.6) and Drug Interactions (7.1)].
Table 17. The Effect of Other Drugs on the Pharmacokinetics of Quetiapine:
| Coadministered Drug | Dose Schedules | Effect on Quetiapine Pharmacokinetics | |
|---|---|---|---|
| Coadministered Drug | Quetiapine | ||
| Phenytoin | 100 mg three times daily | 250 mg three times daily | 5-fold increase in oral clearance |
| Divalproex | 500 mg twice daily | 150 mg twice daily | 17% increase mean max plasma concentration at steady state.No effect on absorption or mean oral clearance |
| Thioridazine | 200 mgtwice daily | 300 mg twice daily | 65% increase in oral clearance |
| Cimetidine | 400 mg three times daily for 4 days | 150 mg three times daily | 20% decrease in mean oral clearance |
| Ketoconazole (potent CYP 3A4 inhibitor) | 200 mg once daily for 4 days | 25 mg single dose | 84% decrease in oral clearance resulting in a 6.2-fold increase in AUC of quetiapine |
| Fluoxetine | 60 mg once daily | 300 mg twice daily | No change in steady state PK |
| Imipramine | 75 mg twice daily | 300 mg twice daily | No change in steady state PK |
| Haloperidol | 7.5 mg twice daily | 300 mg twice daily | No change in steady state PK |
| Risperidone | 3 mg twice daily | 300 mg twice daily | No change in steady state PK |
In vitro enzyme inhibition data suggest that quetiapine and 9 of its metabolites would have little inhibitory effect on in vivo metabolism mediated by cytochromes CYP 1A2, 2C9, 2C19, 2D6, and 3A4. Quetiapine at doses of 750 mg/day did not affect the single dose pharmacokinetics of antipyrine, lithium, or lorazepam (Table 18) [see Drug Interactions (7.2)].
Table 18. The Effect of Quetiapine on the Pharmacokinetics of Other Drugs:
| Coadministered drug | Dose schedules | Effect on other drugs pharmacokinetics | |
|---|---|---|---|
| Coadministered drug | Quetiapine | ||
| Lorazepam | 2 mg, single dose | 250 mg three times daily | Oral clearance of lorazepam reduced by 20% |
| Divalproex | 500 mg twice daily | 150 mg twice daily | Cmax and AUC of free valproic acid at steady-state was decreased by 10-12% |
| Lithium | Up to 2400 mg/day given in twice daily doses | 250 mg three times daily | No effect on steady-state pharmacokinetics of lithium |
| Antipyrine | 1 g, single dose | 250 mg three times daily | No effect on clearance of antipyrine or urinary recovery of its metabolites |
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies were conducted in C57BL mice and Wistar rats. Quetiapine was administered in the diet to mice at doses of 20, 75, 250, and 750 mg/kg and to rats by gavage at doses of 25, 75, and 250 mg/kg for two years. These doses are equivalent to 0.1, 0.5, 1.5, and 4.5 times the MRHD of 800 mg/day based on mg/m² body surface area (mice) or 0.3, 1, and 3 times the MRHD based on mg/m² body surface area (rats). There were statistically significant increases in thyroid gland follicular adenomas in male mice at doses 1.5 and 4.5 times the MRHD based on mg/m² body surface area and in male rats at a dose of 3 times the MRHD on mg/m² body surface area. Mammary gland adenocarcinomas were statistically significantly increased in female rats at all doses tested (0.3, 1, and 3 times the MRHD based on mg/m² body surface area).
Thyroid follicular cell adenomas may have resulted from chronic stimulation of the thyroid gland by thyroid stimulating hormone (TSH) resulting from enhanced metabolism and clearance of thyroxine by rodent liver. Changes in TSH, thyroxine, and thyroxine clearance consistent with this mechanism were observed in subchronic toxicity studies in rat and mouse and in a 1-year toxicity study in rat; however, the results of these studies were not definitive. The relevance of the increases in thyroid follicular cell adenomas to human risk, through whatever mechanism, is unknown.
Antipsychotic drugs have been shown to chronically elevate prolactin levels in rodents. Serum measurements in a 1-year toxicity study showed that quetiapine increased median serum prolactin levels a maximum of 32- and 13-fold in male and female rats, respectively. Increases in mammary neoplasms have been found in rodents after chronic administration of other antipsychotic drugs and are considered to be prolactin-mediated. The relevance of this increased incidence of prolactin-mediated mammary gland tumors in rats to human risk is unknown [see Warnings and Precautions (5.15)].
Mutagenesis
Quetiapine was not mutagenic or clastogenic in standard genotoxicity tests. The mutagenic potential of quetiapine was tested in the in vitro Ames bacterial gene mutation assay and in the in vitro mammalian gene mutation assay in Chinese Hamster Ovary cells. The clastogenic potential of quetiapine was tested in the in vitro chromosomal aberration assay in cultured human lymphocytes and in the in vivo bone marrow micronucleus assay in rats up to 500 mg/kg, which is 6 times the MRHD based on mg/m² body surface area.
Impairment of Fertility
Quetiapine decreased mating and fertility in male Sprague-Dawley rats at oral doses of 50 and 150 mg/kg or approximately 1 and 3 times the MRHD of 800 mg/day based on mg/m² body surface area. Drug-related effects included increases in interval to mate and in the number of matings required for successful impregnation. These effects continued to be observed at 3 times the MRHD even after a two-week period without treatment. The no-effect dose for impaired mating and fertility in male rats was 25 mg/kg, or 0.3 times the MRHD based on mg/m² body surface area. Quetiapine adversely affected mating and fertility in female Sprague-Dawley rats at an oral dose approximately 1 times the MRHD of 800 mg/day based on mg/m² body surface area. Drug-related effects included decreases in matings and in matings resulting in pregnancy, and an increase in the interval to mate. An increase in irregular estrus cycles was observed at doses of 10 and 50 mg/kg, or approximately 0.1 and 1 times the MRHD of 800 mg/day based on mg/m² body surface area. The no-effect dose in female rats was 1 mg/kg, or 0.01 times the MRHD of 800 mg/day based on mg/m² body surface area.
13.2. Animal Toxicology and/or Pharmacology
Quetiapine caused a dose-related increase in pigment deposition in thyroid gland in rat toxicity studies which were 4 weeks in duration or longer and in a mouse 2-year carcinogenicity study. Doses were 10, 25, 50, 75, 150 and 250 mg/kg in rat studies which are approximately 0.1, 0.3, 0.6, 1, 2 and 3-times the MRHD of 800 mg/day based on mg/m² body surface area, respectively. Doses in the mouse carcinogenicity study were 20, 75, 250 and 750 mg/kg which are approximately 0.1, 0.5, 1.5, and 4.5 times the MRHD of 800 mg/day based on mg/m² body surface area. Pigment deposition was shown to be irreversible in rats. The identity of the pigment could not be determined, but was found to be co-localized with quetiapine in thyroid gland follicular epithelial cells. The functional effects and the relevance of this finding to human risk are unknown.
In dogs receiving quetiapine for 6 or 12 months, but not for 1-month, focal triangular cataracts occurred at the junction of posterior sutures in the outer cortex of the lens at a dose of 100 mg/kg, or 4 times the MRHD of 800 mg/day based on mg/m² body surface area. This finding may be due to inhibition of cholesterol biosynthesis by quetiapine. Quetiapine caused a dose-related reduction in plasma cholesterol levels in repeat-dose dog and monkey studies; however, there was no correlation between plasma cholesterol and the presence of cataracts in individual dogs. The appearance of delta-8-cholestanol in plasma is consistent with inhibition of a late stage in cholesterol biosynthesis in these species. There also was a 25% reduction in cholesterol content of the outer cortex of the lens observed in a special study in quetiapine treated female dogs. Drug-related cataracts have not been seen in any other species; however, in a 1-year study in monkeys, a striated appearance of the anterior lens surface was detected in 2/7 females at a dose of 225 mg/kg or 5.5 times the MRHD of 800 mg/day based on mg/m² body surface area.
14. Clinical Studies
14.1 Schizophrenia
Short-term Trials-Adults
The efficacy of SEROQUEL in the treatment of schizophrenia was established in 3 short-term (6-week) controlled trials of inpatients with schizophrenia who met DSM III-R criteria for schizophrenia. Although a single fixed dose haloperidol arm was included as a comparative treatment in one of the three trials, this single haloperidol dose group was inadequate to provide a reliable and valid comparison of SEROQUEL and haloperidol.
Several instruments were used for assessing psychiatric signs and symptoms in these studies, among them the Brief Psychiatric Rating Scale (BPRS), a multi-item inventory of general psychopathology traditionally used to evaluate the effects of drug treatment in schizophrenia. The BPRS psychosis cluster (conceptual disorganization, hallucinatory behavior, suspiciousness, and unusual thought content) is considered a particularly useful subset for assessing actively psychotic schizophrenic patients. A second traditional assessment, the Clinical Global Impression (CGI), reflects the impression of a skilled observer, fully familiar with the manifestations of schizophrenia, about the overall clinical state of the patient.
The results of the trials follow:
- In a 6-week, placebo-controlled trial (n=361) (Study 1) involving 5 fixed doses of SEROQUEL (75 mg/day, 150 mg/day, 300 mg/day, 600 mg/day, and 750 mg/day given in divided doses three times per day), the 4 highest doses of SEROQUEL were generally superior to placebo on the BPRS total score, the BPRS psychosis cluster and the CGI severity score, with the maximal effect seen at 300 mg/day, and the effects of doses of 150 mg/day to 750 mg/day were generally indistinguishable.
- In a 6-week, placebo-controlled trial (n=286) (Study 2) involving titration of SEROQUEL in high (up to 750 mg/day given in divided doses three times per day) and low (up to 250 mg/day given in divided doses three times per day) doses, only the high dose SEROQUEL group (mean dose, 500 mg/day) was superior to placebo on the BPRS total score, the BPRS psychosis cluster, and the CGI severity score.
- In a 6-week dose and dose regimen comparison trial (n=618) (Study 3) involving two fixed doses of SEROQUEL (450 mg/day given in divided doses both twice daily and three times daily and 50 mg/day given in divided doses twice daily), only the 450 mg/day (225 mg given twice daily) dose group was superior to the 50 mg/day (25 mg given twice daily) SEROQUEL dose group on the BPRS total score, the BPRS psychosis cluster, and the CGI severity score.
The primary efficacy results of these three studies in the treatment of schizophrenia in adults is presented in Table 19.
Examination of population subsets (race, gender, and age) did not reveal any differential responsiveness on the basis of race or gender, with an apparently greater effect in patients under the age of 40 years compared to those older than 40. The clinical significance of this finding is unknown.
Adolescents (ages 13-17)
The efficacy of SEROQUEL in the treatment of schizophrenia in adolescents (13–17 years of age) was demonstrated in a 6-week, double-blind, placebo-controlled trial (Study 4). Patients who met DSM-IV diagnostic criteria for schizophrenia were randomized into one of three treatment groups: SEROQUEL 400 mg/day (n=73), SEROQUEL 800 mg/day (n=74), or placebo (n=75). Study medication was initiated at 50 mg/day and on day 2 increased to 100 mg/per day (divided and given two or three times per day). Subsequently, the dose was titrated to the target dose of 400 mg/day or 800 mg/day using increments of 100 mg/day, divided and given two or three times daily. The primary efficacy variable was the mean change from baseline in total Positive and Negative Syndrome Scale (PANSS).
SEROQUEL at 400 mg/day and 800 mg/day was superior to placebo in the reduction of PANSS total score. The primary efficacy results of this study in the treatment of schizophrenia in adolescents is presented in Table 19.
Table 19. Schizophrenia Short-Term Trials:
| Study Number | Treatment Group | Primary Efficacy Endpoint: BPRS Total | ||
| Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo- subtracted Difference* (95% CI) | ||
| Study 1 | SEROQUEL (75 mg/day) | 45.7 (10.9) | -2.2 (2.0) | -4.0 (-11.2, 3.3) |
| SEROQUEL (150 mg/day)† | 47.2 (10.1) | -8.7 (2.1) | -10.4 (-17.8, -3.0) | |
| SEROQUEL (300 mg/day)† | 45.3 (10.9) | -8.6 (2.1) | -10.3 (-17.6, -3.0) | |
| SEROQUEL (600 mg/day)† | 43.5 (11.3) | -7.7 (2.1) | -9.4 (-16.7, -2.1) | |
| SEROQUEL (750 mg/day)† | 45.7 (11.0) | -6.3 (2.0) | -8.0 (-15.2, -0.8) | |
| Placebo | 45.3 (9.2) | 1.7 (2.1) | -- | |
| Study 2 | SEROQUEL (250 mg/day) | 38.9 (9.8) | -4.2 (1.6) | -3.2 (-7.6, 1.2) |
| SEROQUEL (750 mg/day)† | 41.0 (9.6) | -8.7 (1.6) | -7.8 (-12.2, -3.4) | |
| Placebo | 38.4 (9.7) | -1.0 (1.6) | -- | |
| Study 3 | SEROQUEL (450 mg/day BID) | 42.1 (10.7) | -10.0 (1.3) | -4.6 (-7.8, -1.4) |
| SEROQUEL (450 mg/day TID)‡ | 42.7 (10.4) | -8.6 (1.3) | -3.2 (-6.4, 0.0) | |
| SEROQUEL (50 mg BID) | 41.7 (10.0) | -5.4 (1.3) | -- | |
| Primary Efficacy Endpoint: PANSS Total | ||||
| Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference* (95% CI) | ||
| Study 4 | SEROQUEL (400 mg/day)† | 96.2 (17.7) | -27.3 (2.6) | -8.2 (-16.1, -0.3) |
| SEROQUEL (800 mg/day)† | 96.9 (15.3) | -28.4 (1.8) | -9.3 (-16.2, -2.4) | |
| Placebo | 96.2 (17.7) | -19.2 (3.0) | -- | |
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
* Difference (drug minus placebo) in least-squares mean change from baseline.
† Doses that are statistically significant superior to placebo.
‡ Doses that are statistically significant superior to SEROQUEL 50 mg BID.
14.2 Bipolar Disorder
Bipolar I disorder, manic or mixed episodes
Adults
The efficacy of SEROQUEL in the acute treatment of manic episodes was established in 3 placebo-controlled trials in patients who met DSM-IV criteria for bipolar I disorder with manic episodes. These trials included patients with or without psychotic features and excluded patients with rapid cycling and mixed episodes. Of these trials, 2 were monotherapy (12 weeks) and 1 was adjunct therapy (3 weeks) to either lithium or divalproex. Key outcomes in these trials were change from baseline in the Young Mania Rating Scale (YMRS) score at 3 and 12 weeks for monotherapy and at 3 weeks for adjunct therapy. Adjunct therapy is defined as the simultaneous initiation or subsequent administration of SEROQUEL with lithium or divalproex.
The primary rating instrument used for assessing manic symptoms in these trials was YMRS, an 11-item clinician-rated scale traditionally used to assess the degree of manic symptomatology (irritability, disruptive/aggressive behavior, sleep, elevated mood, speech, increased activity, sexual interest, language/thought disorder, thought content, appearance, and insight) in a range from 0 (no manic features) to 60 (maximum score).
The results of the trials follow:
Monotherapy:
The efficacy of SEROQUEL in the acute treatment of bipolar mania was established in 2 placebo-controlled trials. In two 12-week trials (n=300, n=299) comparing SEROQUEL to placebo, SEROQUEL was superior to placebo in the reduction of the YMRS total score at weeks 3 and 12. The majority of patients in these trials taking SEROQUEL were dosed in a range between 400 mg/day and 800 mg per day (studies 1 and 2 in Table 20).
Adjunct Therapy:
In this 3-week placebo-controlled trial, 170 patients with bipolar mania (YMRS ≥20) were randomized to receive SEROQUEL or placebo as adjunct treatment to lithium or divalproex. Patients may or may not have received an adequate treatment course of lithium or divalproex prior to randomization. SEROQUEL was superior to placebo when added to lithium or divalproex alone in the reduction of YMRS total score (Study 3 in Table 20).
The majority of patients in this trial taking SEROQUEL were dosed in a range between 400 mg/day and 800 mg per day. In a similarly designed trial (n=200), SEROQUEL was associated with an improvement in YMRS scores but did not demonstrate superiority to placebo, possibly due to a higher placebo effect.
The primary efficacy results of these studies in the treatment of mania in adults is presented in Table 20.
Children and Adolescents (ages 10-17)
The efficacy of SEROQUEL in the acute treatment of manic episodes associated with bipolar I disorder in children and adolescents (10-17 years of age) was demonstrated in a 3-week, double-blind, placebo-controlled, multicenter trial (Study 4 in Table 20). Patients who met DSM-IV diagnostic criteria for a manic episode were randomized into one of three treatment groups: SEROQUEL 400 mg/day (n=95), SEROQUEL 600 mg/day (n=98), or placebo (n=91). Study medication was initiated at 50 mg/day and on day 2 increased to 100 mg/day (divided doses given two or three times daily). Subsequently, the dose was titrated to a target dose of 400 mg/day or 600 mg/day using increments of 100 mg/day, given in divided doses two or three times daily. The primary efficacy variable was the mean change from baseline in total YMRS score.
SEROQUEL 400 mg/day and 600 mg/day were superior to placebo in the reduction of YMRS total score (Table 20).
Table 20. Mania Trials:
| Study Number | Treatment Group | Primary Efficacy Measure: YMRS Total | ||
| Mean Baseline Score (SD)* | LS Mean Change from Baseline (SE) | Placebo- subtracted Difference† (95% CI) | ||
| Study 1 | SEROQUEL (200-800 mg/day)ठ| 34.0 (6.1) | -12.3 (1.3) | -4.0 (-7.0, -1.0) |
| Haloperidolठ| 32.3 (6.0) | -15.7 (1.3) | -7.4 (-10.4, -4.4) | |
| Placebo | 33.1 (6.6) | -8.3 (1.3) | -- | |
| Study 2 | SEROQUEL (200-800 mg/day)‡ | 32.7 (6.5) | -14.6 (1.5) | -7.9 (-10.9, -5.0) |
| Lithiumठ| 33.3 (7.1) | -15.2 (1.6) | -8.5 (-11.5, -5.5) | |
| Placebo | 34.0 (6.9) | -6.7 (1.6) | -- | |
| Study 3 | SEROQUEL (200-800 mg/day)‡ + mood stabilizer | 31.5 (5.8) | -13.8 (1.6) | -3.8 (-7.1, -0.6) |
| Placebo + mood stabilizer | 31.1 (5.5) | -10 (1.5) | -- | |
| Study 4 | SEROQUEL (400 mg/day)‡ | 29.4 (5.9) | -14.3 (0.96) | -5.2 (-8.1, -2.3) |
| SEROQUEL (600 mg/day)‡ | 29.6 (6.4) | -15.6 (0.97) | -6.6 (-9.5, -3.7) | |
| Placebo | 30.7 (5.9) | -9.0 (1.1) | -- | |
Mood stabilizer: lithium or divalproex; SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
* Adult data mean baseline score is based on patients included in the primary analysis; pediatric mean baseline score is based on all patients in the ITT population.
† Difference (drug minus placebo) in least-squares mean change from baseline.
‡ Doses that are statistically significantly superior to placebo.
§ Included in the trial as an active comparator.
Bipolar Disorder, Depressive Episodes
Adults
The efficacy of SEROQUEL for the acute treatment of depressive episodes associated with bipolar disorder was established in 2 identically designed 8-week, randomized, double-blind, placebo-controlled studies (N=1045) (studies 5 and 6 in Table 21). These studies included patients with either bipolar I or II disorder and those with or without a rapid cycling course. Patients randomized to SEROQUEL were administered fixed doses of either 300 mg or 600 mg once daily.
The primary rating instrument used to assess depressive symptoms in these studies was the Montgomery-Asberg Depression Rating Scale (MADRS), a 10-item clinician-rated scale with scores ranging from 0 to 60. The primary endpoint in both studies was the change from baseline in MADRS score at week 8. In both studies, SEROQUEL was superior to placebo in reduction of MADRS score. Improvement in symptoms, as measured by change in MADRS score relative to placebo, was seen in both studies at Day 8 (week 1) and onwards. In these studies, no additional benefit was seen with the 600 mg dose. For the 300 mg dose group, statistically significant improvements over placebo were seen in overall quality of life and satisfaction related to various areas of functioning, as measured using the Q-LES-Q(SF).
The primary efficacy results of these studies in the acute treatment of depressive episodes associated with bipolar disorder in adults is presented in Table 21.
Table 21. Depressive Episodes Associated with Bipolar Disorder:
| Study Number | Treatment Group | Primary Efficacy Measure: MADRS Total | ||
| Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference* (95% CI) | ||
| Study 5 | SEROQUEL (300 mg/day)† | 30.3 (5.0) | -16.4 (0.9) | -6.1 (-8.3, -3.9) |
| SEROQUEL (600 mg/day)† | 30.3 (5.3) | -16.7 (0.9) | -6.5 (-8.7, -4.3) | |
| Placebo | 30.6 (5.3) | -10.3 (0.9) | -- | |
| Study 6 | SEROQUEL (300 mg/day)† | 31.1 (5.7) | -16.9 (1.0) | -5.0 (-7.3, -2.7) |
| SEROQUEL (600 mg/day)† | 29.9 (5.6) | -16.0 (1.0) | -4.1 (-6.4, -1.8) | |
| Placebo | 29.6 (5.4) | -11.9 (1.0) | -- | |
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
* Difference (drug minus placebo) in least-squares mean change from baseline.
† Doses that are statistically significantly superior to placebo.
Maintenance Treatment as an Adjunct to Lithium or Divalproex
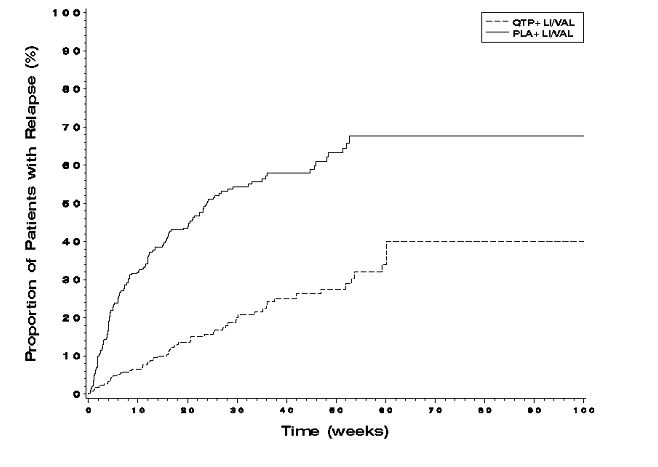
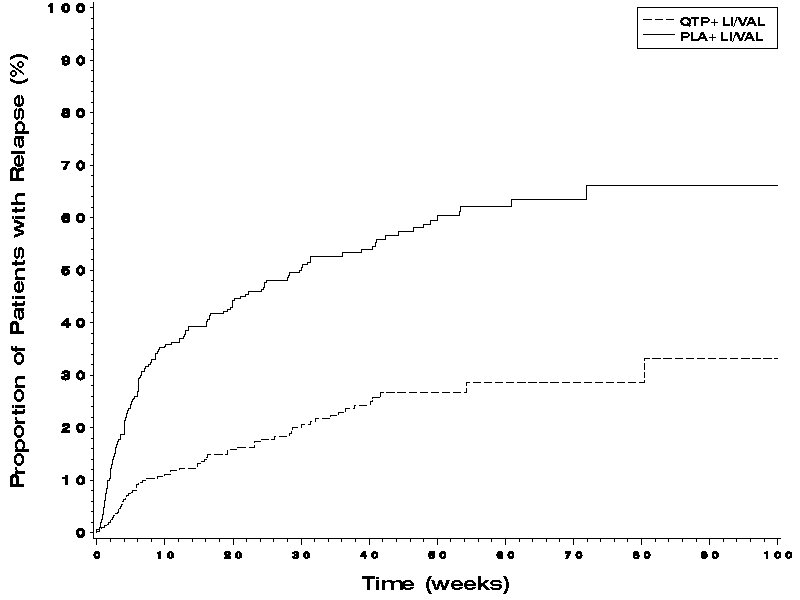
The efficacy of SEROQUEL in the maintenance treatment of bipolar I disorder was established in 2 placebo-controlled trials in patients (n=1326) who met DSM-IV criteria for bipolar I disorder (studies 7 and 8 in Figures 1 and 2). The trials included patients whose most recent episode was manic, depressed, or mixed, with or without psychotic features. In the open-label phase, patients were required to be stable on SEROQUEL plus lithium or divalproex for at least 12 weeks in order to be randomized. On average, patients were stabilized for 15 weeks. In the randomization phase, patients continued treatment with lithium or divalproex and were randomized to receive either SEROQUEL (administered twice daily totaling 400 mg/day to 800 mg/day) or placebo. Approximately 50% of the patients had discontinued from the SEROQUEL group by day 280 and 50% of the placebo group had discontinued by day 117 of double-blind treatment. The primary endpoint in these studies was time to recurrence of a mood event (manic, mixed, or depressed episode). A mood event was defined as medication initiation or hospitalization for a mood episode; YMRS score≥20 or MADRS score≥20 at 2 consecutive assessments; or study discontinuation due to a mood event (Figure 1 and Figure 2).
In both studies, SEROQUEL was superior to placebo in increasing the time to recurrence of any mood event. The treatment effect was present for increasing time to recurrence of both manic and depressed episodes. The effect of SEROQUEL was independent of any specific subgroup (assigned mood stabilizer, sex, age, race, most recent bipolar episode, or rapid cycling course).
Figure 1. Kaplan-Meier Curves of Time to Recurrence of a Mood Event (Study 7):
Figure 2. Kaplan-Meier Curves of Time to Recurrence of a Mood Event (Study 8):
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.