SKYRIZI 75 mg / 150 mg Solution for injection Ref.[8750] Active ingredients: Risankizumab
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: AbbVie Deutschland GmbH & Co. KG, Knollstrasse, 67061, Ludwigshafen, Germany
Pharmacodynamic properties
Pharmacotherapeutic group: Immunosuppressants, interleukin inhibitors
ATC code: L04AC18
Mechanism of action
Risankizumab is a humanised immunoglobulin G1 (IgG1) monoclonal antibody that selectively binds with high affinity to the p19 subunit of human interleukin 23 (IL-23) cytokine without binding to IL-12 and inhibits its interaction with the IL-23 receptor complex. IL-23 is a cytokine that is involved in inflammatory and immune responses. By blocking IL-23 from binding to its receptor, risankizumab inhibits IL-23-dependent cell signalling and release of proinflammatory cytokines.
Pharmacodynamic effects
In a study of subjects with psoriasis, expression of genes associated with the IL-23/IL-17 axis was decreased in the skin after single doses of risankizumab. Reductions in epidermal thickness, infiltration of inflammatory cells, and expression of psoriatic disease markers were also observed in psoriatic lesions.
In a study of subjects with psoriatic arthritis, statistically significant and clinically meaningful reduction from baseline was observed at week 24 in IL-23 and IL-17-associated biomarkers, including serum IL-17A, IL-17F, and IL-22 following treatment with risankizumab 150 mg subcutaneously at week 0, week 4, and every 12 weeks thereafter.
Clinical efficacy and safety
Plaque Psoriasis
The efficacy and safety of risankizumab was assessed in 2 109 subjects with moderate to severe plaque psoriasis in four multicentre, randomised, double-blind studies (ULTIMMA-1, ULTIMMA-2, IMMHANCE, and IMMVENT). Enrolled subjects were 18 years of age and older with plaque psoriasis who had a body surface area (BSA) involvement of ≥10%, a static Physician Global Assessment (sPGA) score of ≥3 in the overall assessment (plaque thickness/induration, erythema, and scaling) of psoriasis on a severity scale of 0 to 4, a Psoriasis Area and Severity Index (PASI) score ≥12, and who were candidates for systemic therapy or phototherapy.
Overall, subjects had a median baseline PASI score of 17.8, a median BSA of 20.0%, and a median baseline DLQI score of 13.0. Baseline sPGA score was severe in 19.3% of subjects and moderate in 80.7% of subjects. A total of 9.8% of study subjects had a history of diagnosed psoriatic arthritis.
Across all studies, 30.9% of subjects were naïve to any systemic therapy (including non-biologic and biologic), 38.1% had received prior phototherapy or photochemotherapy, 48.3% had received prior non-biologic systemic therapy, 42.1% had received prior biologic therapy, and 23.7% had received at least one anti-TNF alpha agent for the treatment of psoriasis. Patients who completed these studies and other Phase ⅔ studies had the opportunity to enrol in an open-label extension study, LIMMITLESS.
ULTIMMA-1 and ULTIMMA-2
ULTIMMA-1 and ULTIMMA-2 enrolled 997 subjects (598 randomised to risankizumab 150 mg, 199 to ustekinumab 45 mg or 90 mg [according to baseline weight], and 200 to placebo). Subjects received treatment at week 0, week 4, and every 12 weeks thereafter. The two co-primary endpoints in ULTIMMA-1 and ULTIMMA-2 were the proportion of subjects who achieved 1) PASI 90 response and 2) sPGA score of clear or almost clear (sPGA 0 or 1) at week 16 versus placebo. The results for the co-primary and other endpoints are presented in Table 2 and Figure 1.
Table 2. Efficacy and quality of life results in adults with plaque psoriasis in ULTIMMA-1 and ULTIMMA-2:
| ULTIMMA-1 | ULTIMMA-2 | |||||
|---|---|---|---|---|---|---|
| Risankizumab (N=304) n (%) | Ustekinumab (N=100) n (%) | Placebo (N=102) n (%) | Risankizumab (N=294) n (%) | Ustekinumab (N=99) n (%) | Placebo (N=98) n (%) | |
| sPGA of clear or almost clear (0 or 1) | ||||||
| Week 16a | 267 (87.8) | 63 (63.0) | 8 (7.8) | 246 (83.7) | 61 (61.6) | 5 (5.1) |
| Week 52 | 262 (86.2) | 54 (54.0) | -- | 245 (83.3) | 54 (54.5) | -- |
| sPGA of clear (0) | ||||||
| Week 16 | 112 (36.8) | 14 (14.0) | 2 (2.0) | 150 (51.0) | 25 (25.3) | 3 (3.1) |
| Week 52 | 175 (57.6) | 21 (21.0) | -- | 175 (59.5) | 30 (30.3) | -- |
| PASI 75 | ||||||
| Week 12 | 264 (86.8) | 70 (70.0) | 10 (9.8) | 261 (88.8) | 69 (69.7) | 8 (8.2) |
| Week 52 | 279 (91.8) | 70 (70.0) | -- | 269 (91.5) | 76 (76.8) | -- |
| PASI 90 | ||||||
| Week 16a | 229 (75.3) | 42 (42.0) | 5 (4.9) | 220 (74.8) | 47 (47.5) | 2 (2.0) |
| Week 52 | 249 (81.9) | 44 (44.0) | -- | 237 (80.6) | 50 (50.5) | -- |
| PASI 100 | ||||||
| Week 16 | 109 (35.9) | 12 (12.0) | 0 (0.0) | 149 (50.7) | 24 (24.2) | 2 (2.0) |
| Week 52 | 171 (56.3) | 21 (21.0) | -- | 175 (59.5) | 30 (30.3) | -- |
| DLQI 0 or 1b | ||||||
| Week 16 | 200 (65.8) | 43 (43.0) | 8 (7.8) | 196 (66.7) | 46 (46.5) | 4 (4.1) |
| Week 52 | 229 (75.3) | 47 (47.0) | -- | 208 (70.7) | 44 (44.4) | -- |
| PSS 0 (symptom-free))c | ||||||
| Week 16 | 89 (29.3) | 15 (15.0) | 2 (2.0) | 92 (31.3) | 15 (15.2) | 0 (0.0) |
| Week 52 | 173 (56.9) | 30 (30.0) | -- | 160 (54.4) | 30 (30.3) | -- |
Figure 1. Time course of mean percent change from baseline of PASI in ULTIMMA-1 and ULTIMMA-2:
RZB = risankizumab
UST = ustekinumab
PBO = placebo
p<0.001 at each time point
Examination of age, gender, race, body weight ≤130 kg, baseline PASI score, concurrent psoriatic arthritis, previous non-biologic systemic treatment, previous biologic treatment, and previous failure of a biologic did not identify differences in response to risankizumab among these subgroups.
Improvements were observed in psoriasis involving the scalp, the nails, and the palms and soles at week 16 and week 52 in subjects treated with risankizumab.
Table 3. Mean changes from baseline in NAPSI, PPASI, and PSS:
| ULTIMMA-1 | ULTIMMA-2 | IMMHANCE | ||||
|---|---|---|---|---|---|---|
| Risankizumab | Placebo | Risankizumab | Placebo | Risankizumab | Placebo | |
| NAPSI: Change at Week 16 (SE) | N=178; -9.0 (1.17) | N=56; 2.1 (1.86)*** | N=177; -7.5 (1.03) | N=49; 3.0 (1.76)*** | N=235; -7.5 (0.89) | N=58; 2.5 (1.70)*** |
| PPASI: Change at Week 16 (SE) | N=95; -5.93 (0.324) | N=34; -3.17 (0.445)*** | N=86; -7.24 (0.558) | N=23; -3.74 (1.025)** | N=113; -7.39 (0.654) | N=26; -0.27 (1.339)*** |
| PSSI: Change at Week 16 (SE) | N=267; -17.6 (0.47) | N=92; -2.9 (0.69)*** | N=252; -18.4 (0.52) | N=83; -4.6 (0.82)*** | N=357; -20.1 (0.40) | N=88; -5.5 (0.77)*** |
| NAPSI: Change at Week 52 (SE) | N=178; -15.7 (0.94) | - | N=183; -16.7 (0.85) | - | - | - |
| PPASI: Change at Week 52 (SE) | N=95; -6.16 (0.296) | - | N=89; -8.35 (0.274) | - | - | - |
| PSSI: Change at Week 52 (SE) | N=269; -17.9 (0.34) | - | N=259; -18.8 (0.24) | - | - | - |
Nail Psoriasis Severity Index (NAPSI), Palmoplantar Psoriasis Severity Index (PPASI), Psoriasis Scalp Severity Index (PSSI), and Standard Error (SE)
** P < 0.01 comparing to risankizumab
*** P < 0.001 comparing to risankizumab
Anxiety and depression, as measured by the Hospital Anxiety and Depression Scale (HADS), improved in the risankizumab group at week 16 compared with the placebo group.
Maintenance of response:
In an integrated analysis of subjects receiving risankizumab in ULTIMMA-1 and ULTIMMA-2 for PASI 100 responders at week 16, 79.8% (206/258) of the subjects who continued on risankizumab maintained the response at week 52. For PASI 90 responders at week 16, 88.4% (398/450) of subjects maintained the response at week 52.
In LIMMITLESS, response rates among subjects who completed ULTIMMA-1 and ULTIMMA-2 and continued risankizumab treatment were maintained through week 160, with 88% (460/525) achieving PASI 90 and 88% (462/525) achieving sPGA response of clear or almost clear.
For subjects who switched from ustekinumab to risankizumab at week 52, rates of PASI 90 and sPGA response of clear or almost clear increased from week 52 through week 76 and were then maintained through week 160.
The safety profile of risankizumab with more than 5 years of exposure was consistent with the profile observed up to 16 weeks.
IMMHANCE
IMMHANCE enrolled 507 subjects (407 randomised to risankizumab 150 mg and 100 to placebo). Subjects received treatment at week 0, week 4, and every 12 weeks thereafter. Subjects who were originally on risankizumab and had a sPGA response of clear or almost clear at week 28 were re-randomised to continue risankizumab every 12 weeks through week 88 (with follow-up 16 weeks after last risankizumab dose) or have treatment withdrawn.
At week 16, risankizumab was superior to placebo on the co-primary endpoints of sPGA of clear or almost clear (83.5% risankizumab vs 7.0% placebo) and PASI 90 (73.2% risankizumab vs 2.0% placebo). Of the 31 subjects from the IMMHANCE study with latent tuberculosis (TB) who did not receive prophylaxis during the study, none developed active TB during the mean follow-up of 55 weeks on risankizumab.
Among subjects with sPGA of clear or almost clear at week 28 in IMMHANCE, 81.1% (90/111) of subjects re-randomised to continued treatment with risankizumab maintained this response at week 104 compared with 7.1% (16/225) who were re-randomised to withdrawal from risankizumab. Of these subjects, 63.1% (70/111) of subjects re-randomised to continued treatment with risankizumab achieved a sPGA clear response at week 104 compared with 2.2% (5/225) who were re-randomised to withdrawal from risankizumab.
Among subjects who achieved sPGA of clear or almost clear at week 28 and relapsed to sPGA of moderate or severe following withdrawal from risankizumab, 83.7% (128/153) regained sPGA of clear or almost clear after 16 weeks of retreatment. Loss of sPGA of clear or almost clear was observed as early as 12 weeks after a missed dose. Of those subjects who were re-randomised to withdraw from treatment, 80.9% (182/225) relapsed, and the median time to relapse was 295 days. No characteristics were identified to predict the time to loss of response or likelihood of regaining response at the individual patient level.
IMMVENT
IMMVENT enrolled 605 subjects (301 randomised to risankizumab and 304 to adalimumab). Subjects randomised to risankizumab received 150 mg of treatment at week 0, week 4, and every 12 weeks thereafter. Subjects randomised to adalimumab received 80 mg at week 0, 40 mg at week 1, and 40 mg every other week through week 15. Starting at week 16, subjects who were receiving adalimumab continued or switched treatment based on response:
- <PASI 50 were switched to risankizumab
- PASI 50 to <PASI 90 were re-randomised to either continue adalimumab or switch to risankizumab
- PASI 90 continued to receive adalimumab
Results are presented in Table 4.
Table 4. Efficacy and quality of life results at week 16 in adults with plaque psoriasis in IMMVENT:
| Risankizumab (N=301) n (%) | Adalimumab (N=304) n (%) | |
|---|---|---|
| sPGA of clear or almost cleara | 252 (83.7) | 183 (60.2) |
| PASI 75 | 273 (90.7) | 218 (71.7) |
| PASI 90a | 218 (72.4) | 144 (47.4) |
| PASI 100 | 120 (39.9) | 70 (23.0) |
| DLQI 0 or 1b | 198 (65.8) | 148 (48.7) |
All comparisons achieved p<0.001
a Co-primary endpoints
b No impact on health-related quality of life
For subjects who had PASI 50 to <PASI 90 with adalimumab at week 16 and were re-randomised, differences in PASI 90 response rates between switching to risankizumab and continuing adalimumab were noted 4 weeks after re-randomisation (49.1% vs 26.8%, respectively).
Results 28 weeks after re-randomisation are presented in Table 5 and Figure 2.
Table 5. Efficacy results 28 weeks after re-randomisation in IMMVENT:
| Switched to Risankizumab (N=53) n (%) | Continued on Adalimumab (N=56) n (%) | |
|---|---|---|
| PASI 90 | 35 (66.0) | 12 (21.4) |
| PASI 100 | 21 (39.6) | 4 (7.1) |
All comparisons achieved p<0.001
Figure 2. Time course of PASI 90 after re-randomisation in IMMVENT:
ADA/ADA: Subjects randomised to adalimumab and continued on adalimumab
ADA/RZB: Subjects randomised to adalimumab and switched to risankizumab
p<0.05 at week 4 and p<0.001 at each time point beginning at week 8
In 270 subjects who switched from adalimumab to risankizumab without a washout period, the safety profile of risankizumab was similar to that in subjects who initiated risankizumab after washout of any prior systemic therapies.
Psoriatic arthritis
Risankizumab has been shown to improve signs and symptoms, physical function, health-related quality of life, and the proportion of subjects with no radiographic progression in adults with active psoriatic arthritis (PsA).
The safety and efficacy of risankizumab were assessed in 1 407 subjects with active PsA in 2 randomised, double-blind, placebo-controlled studies (964 in KEEPSAKE1 and 443 in KEEPSAKE2).
Subjects in these studies had a diagnosis of PsA for at least 6 months based on the Classification Criteria for Psoriatic Arthritis (CASPAR), a median duration of PsA of 4.9 years at baseline, ≥ 5 tender joints and ≥ 5 swollen joints, and active plaque psoriasis or nail psoriasis at baseline. 55.9% of subjects had ≥ 3% BSA with active plaque psoriasis. 63.4% and 27.9% of subjects had enthesitis and dactylitis, respectively. In KEEPSAKE1, where nail psoriasis was further assessed, 67.3% had nail psoriasis.
In both studies, subjects were randomised to receive risankizumab 150 mg or placebo at weeks 0, 4, and 16. Starting from week 28, all subjects received risankizumab every 12 weeks.
In KEEPSAKE1, all subjects had a previous inadequate response or intolerance to non-biologic DMARD therapy and were biologic naïve. In KEEPSAKE2, 53.5% of subjects had a previous inadequate response or intolerance to non-biologic DMARD therapy and 46.5% of subjects had a previous inadequate response or intolerance to biologic therapy.
In both studies, 59.6% of subjects were receiving concomitant methotrexate (MTX), 11.6% were receiving concomitant non-biologic DMARDs other than MTX, and 28.9% were receiving risankizumab monotherapy.
Clinical response
Treatment with risankizumab resulted in significant improvement in measures of disease activity compared with placebo at week 24. For both studies, the primary endpoint was the proportion of subjects who achieved an American College of Rheumatology (ACR) 20 response at week 24. The key efficacy results are shown in Table 6.
Table 6. Efficacy results in studies KEEPSAKE1 and KEEPSAKE2:
| KEEPSAKE1 | KEEPSAKE2 | |||
|---|---|---|---|---|
| Endpoint | Placebo N=481 n (%) | Risankizumab N=483 n (%) | Placebo N=219 n (%) | Risankizumab N=224 n (%) |
| ACR20 Response | ||||
| Week 16 | 161 (33.4) | 272 (56.3)a | 55 (25.3) | 108 (48.3)a |
| Week 24 | 161 (33.5) | 277 (57.3)a | 58 (26.5) | 115 (51.3)a |
| Week 52* | - | 338/433 (78.1) | - | 131/191 (68.6) |
| ACR50 Response | ||||
| Week 24 | 54 (11.3) | 162 (33.4)b | 20 (9.3) | 59 (26.3)b |
| Week 52* | - | 209/435 (48.0) | - | 72/192 (37.5) |
| ACR70 Response | ||||
| Week 24 | 23 (4.7) | 74 (15.3)b | 13 (5.9) | 27 (12.0)c |
| Week 52* | - | 125/437 (28.6) | - | 37/192 (19.3) |
| Resolution of Enthesitis (LEI=0) | ||||
| Week 24* | 156/448 (34.8)d | 215/444 (48.4)a,d | - | - |
| Week 52* | - | 244/393 (62.1)d | - | - |
| Resolution of Dactylitis (LDI=0) | ||||
| Week 24* | 104/204 (51.0)e | 128/188 (68.1)a,e | - | - |
| Week 52* | - | 143/171 (83.6)e | - | - |
| Minimal Disease Activity (MDA) Response | ||||
| Week 24 | 49 (10.2) | 121 (25.0)a | 25 (11.4) | 57 (25.6)a |
| Week 52* | - | 183/444 (41.2) | - | 61/197 (31.0) |
* data are shown for available subjects in the format of n/N observed (%)
a multiplicity-controlled p≤0.001 risankizumab vs placebo comparison.
b nominal p≤0.001 risankizumab vs placebo comparison.
c nominal p≤0.05 risankizumab vs placebo comparison.
d Summarized from pooled data from KEEPSAKE1 and KEEPSAKE2 for subjects with baseline LEI >0.
e Summarized from pooled data from KEEPSAKE1 and KEEPSAKE2 for subjects with baseline LDI >0.
Response over time:
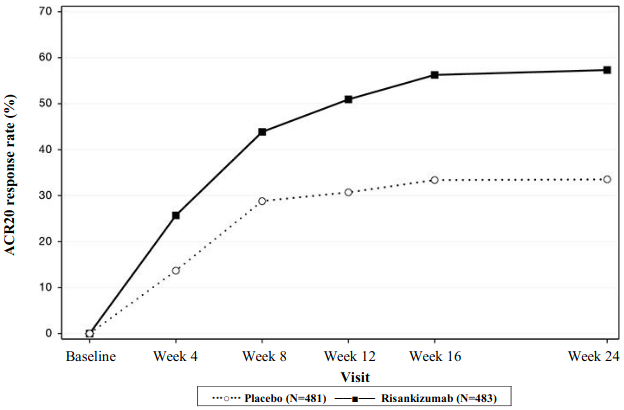
In KEEPSAKE1, a greater ACR20 response was observed in the risankizumab group compared to placebo as early as week 4 (25.7%) and the treatment difference continued over time to week 24 (Figure 3).
Figure 3. Percent of subjects achieving ACR20 responses in study KEEPSAKE1 through week 24:
A greater ACR20 response for risankizumab versus placebo was seen as early as week 4 in 19.6% of subjects in KEEPSAKE2.
Responses observed in risankizumab groups were similar regardless of concomitant non-biologic DMARD use, number of prior non-biologic DMARDs, age, gender, race, and BMI. In KEEPSAKE2, responses were seen regardless of prior biologic therapy.
The safety profile of risankizumab with up to 52 weeks of exposure was consistent with the profile observed up to 24 weeks.
In both studies, the proportion of subjects achieving modified PsA Response Criteria (PsARC) at week 24 was higher in subjects receiving risankizumab compared with placebo. In addition, subjects receiving risankizumab achieved greater improvement in Disease Activity Score (28 joints) using CRP (DAS28-CRP) compared with placebo at week 24. Improvements were maintained through week 52 for PsARC and DAS28-CRP.
Treatment with risankizumab resulted in improvements in individual ACR components, Health Assessment Questionnaire-Disability Index (HAQ-DI), pain assessment, and high-sensitivity C-reactive protein (hsCRP) compared with placebo.
Treatment with risankizumab resulted in statistically significant improvement in the skin manifestations of psoriasis in subjects with PsA.
Treatment with risankizumab resulted in statistically significant improvement in the modified Nail Psoriasis Severity Index (mNAPSI) and the 5-point Physician's Global Assessment of Fingernail Psoriasis (PGA-F) scores in subjects with nail psoriasis at baseline (67.3%) in KEEPSAKE1. This improvement was maintained through week 52 (see Table 7).
Table 7. Nail psoriasis efficacy results in KEEPSAKE1:
| Placebo N=338 | Risankizumab N=309 | |
|---|---|---|
| mNAPSI change from baselinea | ||
| Week 24 | -5.57 | -9.76b |
| Week 52 | - | -13.64 |
| PGA-F change from baselinea | ||
| Week 24 | -0.4 | -0.8b |
| Week 52 | - | -1.2 |
| PGA-F clear/minimal and ≥2-grade improvementc | ||
| Week 24 n (%) | 30 (15.9) | 71 (37.8)d |
| Week 52 n (%) | - | 105 (58.0) |
a Summarized for subjects with baseline nail psoriasis (Placebo N=338; risankizumab N=309; at week 52, for mNAPSI, observed risankizumab N=290, for PGA-F, observed risankizumab N=291).
b Multiplicity-controlled p≤0.001 risankizumab vs placebo comparison.
c Summarized for subjects with nail psoriasis and a PGA-F overall global assessment score of 'Mild', 'Moderate' or 'Severe' at Baseline (Placebo N=190; risankizumab N=188, at week 52 observed risankizumab N=181).
d Nominal p≤0.001 risankizumab vs placebo comparison.
Radiographic response
In KEEPSAKE1, inhibition of progression of structural damage was assessed radiographically and expressed as the change in modified Total Sharp Score (mTSS) at week 24, compared with baseline. The mTSS score was modified for PsA by addition of hand distal interphalangeal (DIP) joints. At week 24, the mean progression of structural damage with risankizumab (mean mTSS 0.23) compared with placebo (mean mTSS 0.32) was not statistically significant. At week 24, the proportion of subjects with no radiographic progression (defined as a change from baseline in mTSS ≤ 0) was higher with risankizumab (92.4%) compared with placebo (87.7%). This response was maintained through week 52.
Physical function and health related quality of life
In both studies, subjects treated with risankizumab showed statistically significant improvement from baseline in physical function as assessed by HAQ-DI at week 24 (KEEPSAKE1 (-0.31) compared with placebo (-0.11) (p ≤0.001)), (KEEPSAKE2 (-0.22) compared with placebo (-0.05) (p ≤0.001)). At week 24, a greater proportion of subjects achieved a clinically meaningful reduction of at least 0.35 in HAQ-DI score from baseline in the risankizumab group compared with placebo. Improvements in physical function were maintained through week 52.
In both studies, subjects treated with risankizumab demonstrated significant improvements in the SF-36 V2 physical component summary scores and in FACIT-Fatigue scores at week 24, compared with placebo, with improvements maintained through week 52.
At baseline, psoriatic spondylitis was reported in 19.6% (7.9% diagnosed by radiograph or MRI) of subjects in KEEPSAKE1 and 19.6% (5% diagnosed by radiograph or MRI) of subjects in KEEPSAKE2. Subjects with clinically assessed psoriatic spondylitis who were treated with risankizumab showed improvements from baseline in Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) scores compared with placebo at week 24. Improvements were maintained through week 52. There is insufficient evidence of the efficacy of risankizumab in subjects with radiograph- or MRI-confirmed ankylosing spondylitis-like psoriatic arthropathy due to the small number of subjects studied.
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with Skyrizi in one or more subsets of the paediatric population in the treatment of plaque psoriasis and psoriatic arthritis (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
The pharmacokinetics of risankizumab was similar between subjects with plaque psoriasis and subjects with psoriatic arthritis.
Absorption
Risankizumab exhibited linear pharmacokinetics with dose-proportional increase in exposure across dose ranges of 18 to 300 mg and 0.25 to 1 mg/kg administered subcutaneously, and 200 to 1 200 mg and 0.01 to 5 mg/kg administered intravenously.
Following subcutaneous dosing of risankizumab, peak plasma concentrations were achieved between 3-14 days after dosing with an estimated absolute bioavailability of 89%. With dosing of 150 mg at week 0, week 4, and every 12 weeks thereafter, estimated steady-state peak and trough plasma concentrations are 12 and 2 μg/mL, respectively.
Bioequivalence was demonstrated between a single risankizumab 150 mg injection and two risankizumab 75 mg injections in pre-filled syringe. Bioequivalence was also demonstrated between risankizumab 150 mg pre-filled syringe and pre-filled pen.
Distribution
The mean (±standard deviation) steady-state volume of distribution (Vss) of risankizumab was 11.4 (±2.7) L in Phase 3 studies in subjects with psoriasis, indicating that the distribution of risankizumab is primarily confined to the vascular and interstitial spaces.
Biotransformation
Therapeutic IgG monoclonal antibodies are typically degraded into small peptides and amino acids via catabolic pathways in the same manner as endogenous IgGs. Risankizumab is not expected to be metabolised by cytochrome P450 enzymes.
Elimination
The mean (±standard deviation) systemic clearance (CL) of risankizumab was 0.3 (±0.1) L/day in Phase 3 studies in subjects with psoriasis. The mean terminal elimination half-life of risankizumab ranged from 28 to 29 days in Phase 3 studies in subjects with psoriasis.
As an IgG1 monoclonal antibody, risankizumab is not expected to be filtered by glomerular filtration in the kidneys or to be excreted as an intact molecule in the urine.
Linearity/non-linearity
Risankizumab exhibited linear pharmacokinetics with approximately dose-proportional increases in systemic exposure (Cmax and AUC) in the evaluated dose ranges of 18 to 300 mg or 0.25 to 1 mg/kg subcutaneous administration in healthy subjects or subjects with psoriasis.
Interactions
An interaction study was conducted in subjects with plaque psoriasis to assess the effect of repeated administration of risankizumab on the pharmacokinetics of cytochrome P450 (CYP) sensitive probe substrates. The exposure of caffeine (CYP1A2 substrate), warfarin (CYP2C9 substrate), omeprazole (CYP2C19 substrate), metoprolol (CYP2D6 substrate) and midazolam (CYP3A substrate) following risankizumab treatment were comparable to their exposures prior to risankizumab treatment, indicating no clinically meaningful interactions through these enzymes.
Population pharmacokinetic analyses indicated that risankizumab exposure was not impacted by concomitant treatment used by some subjects with plaque psoriasis or psoriatic arthritis during the clinical studies.
Special populations
Paediatric population
The pharmacokinetics of risankizumab in paediatric subjects has not been established.
Elderly
Of the 2 234 subjects with plaque psoriasis exposed to risankizumab, 243 were 65 years or older and 24 subjects were 75 years or older. Of the 1 542 subjects with psoriatic arthritis exposed to risankizumab, 246 were 65 years or older and 34 subjects were 75 years or older. No overall differences in risankizumab exposure were observed between older and younger subjects who received risankizumab.
Patients with renal or hepatic impairment
No specific studies have been conducted to determine the effect of renal or hepatic impairment on the pharmacokinetics of risankizumab. Based on population pharmacokinetic analyses, serum creatinine levels, creatinine clearance, or hepatic function markers (ALT/AST/bilirubin) did not have a meaningful impact on risankizumab clearance in subjects with plaque psoriasis or psoriatic arthritis.
As an IgG1 monoclonal antibody, risankizumab is mainly eliminated via intracellular catabolism and is not expected to undergo metabolism via hepatic cytochrome P450 enzymes or renal elimination.
Body weight
Risankizumab clearance and volume of distribution increase as body weight increases which may result in reduced efficacy in subjects with high body weight (>130 kg). However, this observation is based on a limited number of subjects. No dose adjustment based on body weight is currently recommended.
Gender or race
The clearance of risankizumab was not significantly influenced by gender or race in adult subjects with plaque psoriasis or psoriatic arthritis. No clinically meaningful differences in risankizumab exposure were observed in Chinese or Japanese subjects compared to Caucasian subjects in a clinical pharmacokinetic study in healthy volunteers.
Preclinical safety data
Nonclinical data revealed no special hazard for humans based on repeat-dose toxicity studies including safety pharmacology evaluations, and an enhanced pre- and post-natal developmental toxicity study in cynomolgus monkeys at doses of up to 50 mg/kg/week (producing exposures of about 70 times the clinical exposure at maximum recommended human dose [MRHD]).
Mutagenicity and carcinogenicity studies have not been conducted with risankizumab. In a 26-week chronic toxicology study in cynomolgus monkeys at doses of up to 50 mg/kg/week (about 70 times the clinical exposure at the MRHD), there were no pre-neoplastic or neoplastic lesions observed and no adverse immunotoxicity or cardiovascular effects were noted.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.