SKYTROFA Powder and solvent for solution for injection Ref.[28359] Active ingredients: Lonapegsomatropin
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Ascendis Pharma Endocrinology Division A/S, Tuborg Boulevard 12, DK-2900 Hellerup, Denmark
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Pituitary and hypothalamic hormones and analogues, somatropin and somatropin agonists
ATC Code: H01AC09
Mechanism of action
Lonapegsomatropin is a long-acting 'prodrug' of somatropin. Lonapegsomatropin consists of the parent drug, somatropin, that is transiently conjugated to a methoxypolyethylene glycol carrier (4 x 10 kDa mPEG) via a proprietary TransCon Linker. The carrier has a shielding effect that minimizes renal excretion and receptor-mediated clearance of lonapegsomatropin. After subcutaneous administration, lonapegsomatropin releases fully active somatropin via autocleavage of the TransCon Linker. Somatropin (191 amino acids) has the same mode of action and distribution as daily somatropin, but with a once-weekly subcutaneous injection.
Somatropin binds to a dimeric hGH receptor in the cell membrane of target cells resulting in intracellular signal transduction and a host of pharmacodynamic effects. Somatropin has direct tissue and metabolic effects, and indirect effects mediated by IGF-1, including stimulation of chondrocyte differentiation and proliferation, stimulation of hepatic glucose output, protein synthesis and lipolysis. Somatropin stimulates skeletal growth in paediatric patients with GHD as a result of effects on the growth plates (epiphyses) of bones.
Pharmacodynamic effects
Somatropin released from lonapegsomatropin produces a dose linear IGF-1 response, with a change in dose of 0.02 mg somatropin/kg resulting in an approximate change in average weekly IGF-1 standard deviation score (SDS) of 0.17.
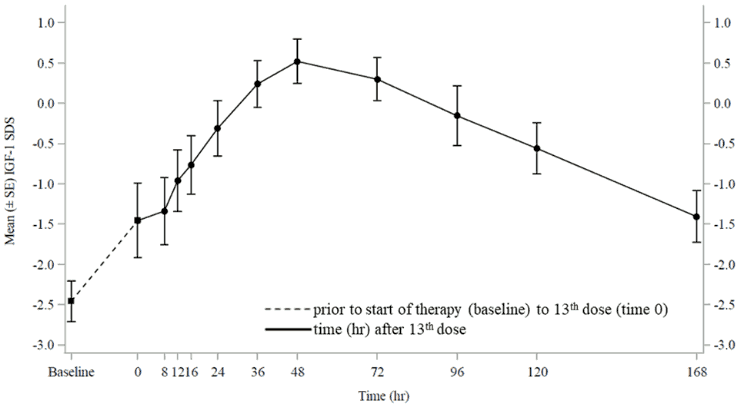
At steady-state, IGF-1 SDS levels peaked approximately 2 days post-dose, with the average weekly IGF-1 SDS coinciding with approximately 4.5 days post-dose (Figure 1). IGF-1 SDS levels were in the normal range for GHD patients for the majority of the week, similar to daily somatropin.
Figure 1. Mean (±SE) IGF-1 SDS at steady-state in children with GHD after administration of once-weekly lonapegsomatropin 0.24 mg somatropin/kg/week:
Clinical efficacy and safety
The efficacy and safety of once-weekly lonapegsomatropin were evaluated in phase 3 clinical trials that included 306 paediatric patients with GHD.
In a 52-week multi-centre randomised, open-label, active-controlled, parallel-group phase 3 clinical trial, 161 treatment-naïve, prepubertal paediatric patients with GHD were randomised to once-weekly lonapegsomatropin (N=105) or daily somatropin (N=56), both at a total weekly dose of 0.24 mg somatropin/kg. The patients ranged in age from 3.2 to 13.1 years with a mean of 8.5 years. Most (N=132 (82%)) subjects were male. The patients had a mean baseline height SDS of -2.93. The primary efficacy endpoint was annualised height velocity (AHV) at week 52. Treatment with once-weekly lonapegsomatropin for 52 weeks resulted in a non-inferior AHV compared to once-daily somatropin (Table 4). Also, changes in the height standard deviation score (SDS) (change from baseline) tended to be larger for once-weekly lonapegsomatropin compared to once-daily somatropin (Table 4). Changes in AHV and height SDS tended to be larger for lonapegsomatropin compared to those of somatropin from week 26 through the end of the trial at week 52.
The mean (SD) ratio of bone age to chronological age advanced similarly in both arms from baseline to week 52: 0.69 (0.16) to 0.75 (0.15) with once-weekly lonapegsomatropin and 0.70 (0.14) to 0.76 (0.14) with daily somatropin.
Table 4. Growth and IGF-1 response at week 52 in paediatric treatment-naïve patients with GHD (Intention-to-treat analysis):
| Once-weekly lonapegsomatropin (N=105) (0.24 mg somatropin/kg/week) | Daily somatropin (N=56) (0.24 mg somatropin/kg/week) | Estimate of treatment difference (lonapegsomatropin minus somatropin) | |
|---|---|---|---|
| AHV (cm/year)a, LS mean (95% CI) | 11.2 (10.7-11.6) | 10.3 (9.7-10.9) | 0.9b (0.2-1.5) |
| Height SDS, change from baselinec, LS mean (95% CI) | 1.10 (1.02-1.18) | 0.96 (0.85-1.06) | 0.14d (0.03-0.26) |
| IGF-1 SDS categorye, % <0 0 to +2 +2 to +3 >+3 | 23.1% 69.2% 7.7% 0 | 40.7% 57.4% 1.9% 0 | Not analysed |
a AHV: The estimates of LS mean and 95% CI are from an ANCOVA model that included baseline age, peak growth hormone levels (log transformed) at stimulation test, baseline height SDS – average SDS of parental height as covariates, and treatment and gender as factors. Missing data are imputed with multiple imputation method.
b p=0.0088 (2-sided) for superiority
c Height SDS, change from baseline: The estimates of LS mean and 95% CI are from an ANCOVA model that included baseline age, peak growth hormone levels (log transformed) at stimulation test and baseline height SDS as covariates, and treatment and gender as factors.
d p=0.0149 (2-sided)
e Average level at week 52
In an open-label extension period, patients who continued treatment with lonapegsomatropin had an increase in height SDS of 1.61 from baseline to week 104. Patients who switched from daily somatropin to lonapegsomatropin at week 52 had an increase in height SDS of 1.49 from baseline to week 104.
Supportive evidence
Evidence from additional clinical trials on lonapegsomatropin support the long-term clinical efficacy of lonapegsomatropin treatment.
In a 26-week single-arm open-label clinical trial evaluating lonapegsomatropin 0.24 mg somatropin/kg/week in 146 paediatric GHD patients aged 1 to 17 years old, of whom 143 had received prior daily somatropin treatment for mean (SD) 1.1 (0.7) years, the mean (SD) annualized height velocity was 9 (2.7) cm/year and the mean (SD) change from trial baseline in height SDS was 0.28 (0.25). Patient and caregiver preference were evaluated at week 13. 84% of patients and 90% of caregivers preferred once-weekly lonapegsomatropin over their prior daily somatropin.
Table 5. Average IGF-1 SDS levels at baseline and week 26 in paediatric treatment-experienced patients with GHD (intention-to-treat analysis):
| Average IGF-1 SDS category | Baseline (N=143) n (%) | Week 26 (N=139) n (%) |
|---|---|---|
| <0 | 37 (25.9) | 13 (9.4) |
| 0 to +2 | 74 (51.7) | 71 (51.1) |
| +2 to +3 | 27 (18.9) | 33 (23.7) |
| > +3 | 5 (3.5) | 22 (15.8) |
5.2. Pharmacokinetic properties
The pharmacokinetics following administration of lonapegsomatropin was assessed after single dose in a total of 73 healthy adults in 2 trials. In addition, PK in paediatrics with GHD was evaluated based on intense sampling at week 13 in 11 subjects and sparse sampling in 109 subjects across 2 trials. Demographic details are provided in Table 6 for the subjects included in the pharmacokinetic evaluation of lonapegsomatropin.
Table 6. Demography of subjects in pharmacokinetic evaluation of lonapegsomatropin:
| Category | Healthy adults | Children with GHD |
|---|---|---|
| N | 73 | 109 |
| Male / Female | 55 / 19 | 87 / 22 |
| American Indian or Alaska Native | 0 | 0 |
| Asian | 10 | 1 |
| Black or African American | 13 | 2 |
| Native Hawaiian or Other Pacific Islander | 0 | 0 |
| White | 49 | 104 (11 with intense PK sampling) |
| Other/Multiple | 1 | 2 |
| Hispanic or Latino | 23 | 5 |
| Not Hispanic or Latino | 50 | 104 |
Absorption
Following subcutaneous dose administration, lonapegsomatropin releases somatropin in a controlled manner that follows first-order kinetics.
In paediatric GHD patients, following subcutaneous dose administration of lonapegsomatropin 0.24 mg somatropin/kg/week, the observed mean (CV%) steady state peak serum concentration (Cmax) of lonapegsomatropin was 1230 (86.3) ng somatropin/mL at median Tmax of 25 hours, and for released somatropin Cmax was 15.2 (83.4) ng/mL with a median time to reach Cmax of 12 hours. The mean (CV%) somatropin exposure over the one-week dose interval (area under the curve) was 500 (83.8) h*ng/mL. Accumulation of lonapegsomatropin or somatropin following repeat dose administration was not observed.
In paediatric GHD patients, injections were rotated between the abdomen, buttock, and thigh. No apparent association of administration site with somatropin exposure was observed.
The absolute bioavailability of lonapegsomatropin following subcutaneous dose administration has not been investigated.
Distribution
In paediatric GHD patients, the mean (CV%) steady state apparent volume of distribution of lonapegsomatropin after subcutaneous administration of 0.24 mg somatropin/kg/week was 0.13 (109) L/kg. Somatropin released from lonapegsomatropin is expected to have a similar volume of distribution as endogenous growth hormone.
Elimination
Metabolism
The metabolic fate of somatropin involves protein catabolism in both the liver and kidneys.
Excretion
In paediatric GHD patients, the mean (CV%) steady state apparent clearance of lonapegsomatropin after subcutaneous administration of 0.24 mg somatropin/kg/week was 3.2 (67) mL/h/kg with a mean (±SD) observed half-life of 30.7 (± 12.7) hours. The apparent half-life of somatropin released from lonapegsomatropin was approximately 25 hours.
Special populations
No sex-specific pharmacokinetic studies have been done with lonapegsomatropin. The available literature indicates that the pharmacokinetics of somatropin is similar in males and females.
Based on a population pharmacokinetic analysis, age, sex, race/ethnicity, and body weight do not have a clinically meaningful effect on the pharmacokinetics.
No studies in patients with renal or hepatic impairments have been conducted with lonapegsomatropin (see section 4.2). A reduction in somatropin clearance following administration of daily somatropin has been noted in patients with severe liver and kidney dysfunction. The clinical significance of this decrease is unknown. The pharmacokinetics of the mPEG carrier of lonapegsomatropin is expected to be dependent on renal function but has not been assessed in patients with renal impairment.
Lonapegsomatropin has not been studied in patients below 6 months of age (see section 4.2).
5.3. Preclinical safety data
Non-clinical data reveal no special hazard for humans based on safety pharmacology, repeated dose toxicity, genotoxicity, and carcinogenicity.
Reproductive toxicology studies performed in rats and histopathological evaluation of reproductive organs in monkeys administered subcutaneous lonapegsomatropin at doses up to 20-fold the clinical dose of 0.24 mg somatropin/kg/week did not induce adverse effects on male and female fertility or on reproductive organs. Due to antibody formation impairing exposure in rats, no firm conclusion can be made with respect to the relevance for human fertility.
No embryonic or foetal development toxicities occurred in rats administered subcutaneous lonapegsomatropin at doses up to 13-fold the clinical dose of 0.24 mg somatropin/kg/week. Due to intermittent exposure no firm conclusion can be made with respect to the embryo-foetal development study in rats.
An embryo-foetal development toxicity study in rabbits has shown foetal abnormalities and embryo-foetal mortality at 1.5-fold and 6-fold, the clinical dose of 0.24 mg somatropin/kg/week, respectively, and possibly caused by maternal toxicity. The clinical relevance of these findings is uncertain.
In a pre- and postnatal developmental study in rats there were no adverse effects on the pregnant/lactating female or on development of the conceptus and the offspring following exposure of the female from implantation through weaning to subcutaneous doses of a structurally related transiently pegylated somatropin prodrug up to 13-fold the clinical dose of 0.24 mg somatropin/kg/week.
mPEG exposure
At about 10 times the human exposure to the mPEG component of lonapegsomatropin, vacuolation occurs in choroid plexus (CP) epithelial cells of cynomolgus monkeys after one year of exposure. At about 34 times the human exposure to mPEG, a slight increase in the number of animals with vacuoles was seen in CP epithelial cells of monkeys. The vacuolation was not associated with adverse morphological changes or clinical signs. Vacuolation of cells is considered an adaptive response. Therefore, this is not considered as a possible adverse effect in humans at the therapeutic dose.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.