SPEXOTRAS Powder for oral solution Ref.[107937] Active ingredients: Trametinib
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road, Dublin 4, Ireland
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, protein kinase inhibitors, mitogen-activated protein kinase (MEK) inhibitors
ATC code: L01EE01
Mechanism of action
Trametinib is a reversible, highly selective, allosteric inhibitor of mitogen-activated extracellular signal regulated kinase 1 (MEK1) and MEK2 activation and kinase activity. MEK proteins are components of the extracellular signal-related kinase (ERK) pathway. In human cancers, this pathway is often activated by mutated forms of BRAF which activates MEK. Trametinib inhibits activation of MEK by BRAF and inhibits MEK kinase activity.
Combination with dabrafenib
Dabrafenib is an inhibitor of RAF kinases. Oncogenic mutations in BRAF lead to constitutive activation of the RAS/RAF/MEK/ERK pathway.
Thus, trametinib and dabrafenib inhibit two kinases in this pathway, MEK and RAF, and therefore the combination provides concomitant inhibition of the pathway. The combination of trametinib with dabrafenib has shown anti-tumour activity in BRAF V600 mutation-positive cancer cell lines in vitro and delays the emergence of resistance in vivo in BRAF V600 mutation-positive xenografts.
Clinical efficacy and safety
Paediatric population
The clinical efficacy and safety of dabrafenib plus trametinib combination therapy in paediatric patients aged 1 to <18 years with BRAF V600 mutation-positive glioma was evaluated in a multicentre, open-label, Phase II clinical study (EudraCT 2015-004015-20). Patients with low-grade glioma (WHO 2016 Grades 1 and 2) who required first systemic therapy were randomised in a 2:1 ratio to dabrafenib plus trametinib or carboplatin plus vincristine, and patients with relapsed or refractory high-grade glioma (WHO 2016 Grades 3 and 4) were enrolled into a single-arm dabrafenib plus trametinib cohort.
BRAF mutation status was identified prospectively in tumour tissue via a local test, or by a central laboratory using the bioMérieux THxID-BRAF kit when a local test was not available. In addition, retrospective testing of available tumour samples by the central laboratory was performed to confirm the BRAF V600E mutation.
Dabrafenib and trametinib dosing in the clinical study was age- and weight-dependent, with dabrafenib dosed orally at 2.625 mg/kg twice daily for ages <12 years and at 2.25 mg/kg twice daily for ages 12 years and older; trametinib was dosed orally at 0.032 mg/kg once daily for ages <6 years and at 0.025 mg/kg once daily for ages 6 years and older. Dabrafenib doses were capped at 150 mg twice daily and trametinib doses at 2 mg once daily. Carboplatin and vincristine were dosed based on age and body surface area at doses of 175 mg/m² and 1.5 mg/m², respectively, as weekly infusions. Carboplatin and vincristine were administered in one 10-week induction course followed by eight 6-week cycles of maintenance therapy.
The primary efficacy endpoint in both cohorts was overall response rate (ORR, sum of confirmed complete/CR and partial responses/PR) by independent review based on RANO (2017) criteria for the LGG cohort, and RANO (2010) criteria for the HGG cohort. The primary analysis was performed when all patients in both cohorts had completed at least 32 weeks of therapy.
BRAF mutation-positive paediatric low-grade glioma (WHO Grades 1 and 2)
In the low-grade glioma cohort, 110 patients were randomised to dabrafenib plus trametinib (n=73) or carboplatin plus vincristine (n=37). Median age was 9.5 years, with 34 patients (30.9%) aged 12 months to <6 years, 36 patients (32.7%) aged 6 to <12 years and 40 patients (36.4%) aged 12 to <18 years; 60% were female. The majority of patients (80%) had Grade 1 glioma at initial diagnosis. The most common pathologies were pilocytic astrocytoma (30.9%), ganglioglioma (27.3%) and LGG not otherwise specified (NOS) (18.2%). Metastatic sites were present in 9 patients (8.2%). Prior surgery was reported in 91 patients (82.7%), among those patients the procedure at last surgery was resection in 28 patients (25.5%). Systemic corticosteroid use was reported in 36 patients (32.7%).
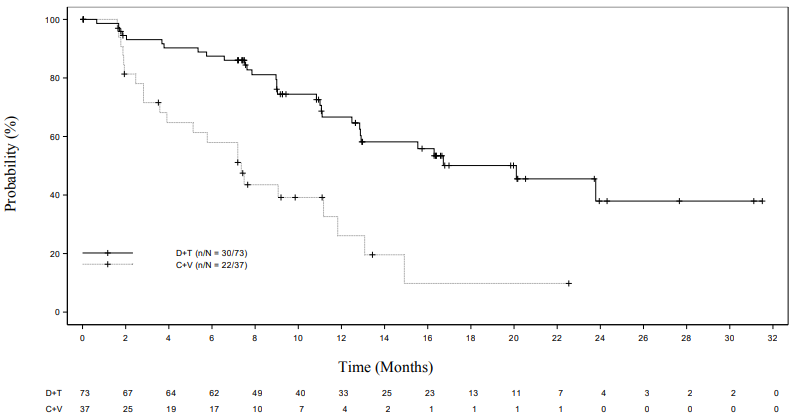
The ORR in the dabrafenib plus trametinib arm showed a statistically significant improvement over carboplatin plus vincristine. The subsequent hierarchical testing also demonstrated a statistically significant improvement in progression-free survival (PFS) over chemotherapy (Table 6).
At the time of the primary analysis, conducted after all patients had completed at least 32 weeks of treatment or had discontinued earlier, the overall survival (OS) data were still immature (one death was reported in the carboplatin plus vincristine (C+V) arm).
Table 6. Response and progression-free survival in the pivotal study G2201 (LGG cohort, primary analysis):
| Dabrafenib + Trametinib (D+T) N=73 | Carboplatin + Vincristine (C+V) N=37 | |
|---|---|---|
| Best overall response | ||
| Complete response (CR), n (%) | 2 (2.7) | 1 (2.7) |
| Partial response (PR), n (%) | 32 (43.8) | 3 (8.1) |
| Stable disease (SD), n (%) | 30 (41.1) | 15 (40.5) |
| Progressive disease (PD), n (%) | 8 (11.0) | 12 (32.4) |
| Unknown, n (%) | 1 (1.4) 6 | (16.2)1 |
| Overall response rate | ||
| ORR (CR+PR), 95% CI | 46.6% (34.8 – 58.6%) | 10.8% (3.0 – 25.4%) |
| Odds ratio2 | 7.19 (2.3 – 22.4), p<0.001 | |
| Risk difference | 35.8% (20.6 – 51.0) | |
| Progression-free survival (PFS) | ||
| PFS (months), (95% CI) | 20.1 (12.8 – NE) | 7.4 (3.6 – 11.8) |
| Hazard ratio (95% CI), p-value | 0.31 (0.17 – 0.55), p<0.001 | |
NE = not evaluable
1 4 patients randomised to C+V discontinued prior to receiving treatment.
2 Odds ratio (D+T vs C+V) and 95% CI are from a logistic regression with treatment as the only covariate, i.e. it is the odds of observing a response in the D+T arm compared to the odds of observing a response in the C+V arm. Odds ratio >1 favours D+T.
Figure 1. Kaplan-Meier curves for progression-free survival in the pivotal study G2201 (LGG cohort, primary analysis):
BRAF mutation-positive paediatric high-grade glioma (WHO Grades 3 and 4)
In the single-arm high-grade glioma cohort, 41 patients with relapsed or refractory HGG were enrolled and treated with dabrafenib plus trametinib for a median duration of 72.7 weeks. Median age was 13.0 years, with 5 patients (12.2%) aged 12 months to <6 years, 10 patients (24.4%) aged 6 to <12 years and 26 patients (63.4%) aged 12 to <18 years; 56% were female. The histological grade at initial diagnosis was Grade 4 in 20 patients (48.8%), Grade 3 in 13 patients (31.7%), Grade 2 in 4 patients (9.8%), Grade 1 in 3 patients (7.3%) and missing in 1 patient (2.4%). The most common pathologies were glioblastoma multiforme (31.7%), anaplastic pleomorphic xanthoastrocytoma (14.6%), HGG NOS (9.8%) and pleomorphic xanthoastrocytoma (9.8%). Prior surgery was reported in 40 patients (97.6%), among those patients the procedure at last surgery was resection in 24 patients (58.5%). Prior antineoplastic chemotherapy was reported for 33 patients (80.5%). Prior radiotherapy was reported for 37 patients (90.2%). Systemic corticosteroid use while on study treatment was reported in 21 patients (51.2%).
The ORR in this cohort was 56.1% (23/41), 95% CI (39.7%, 71.5%): CR in 12 patients (29.3%) and PR in 11 patients (26.8%). The median duration of response (DOR) was 22.2 months (95% CI: 7.6 – NE), with 15 patients (65.2%) censored at the time of the primary analysis.
5.2. Pharmacokinetic properties
The pharmacokinetic properties of trametinib have mostly been determined in adult patients using the solid (tablet) formulation. The pharmacokinetics of trametinib following single or repeat weight-adjusted dosing were also evaluated in 244 paediatric patients. Pharmacokinetic characteristics (drug absorption rate and drug clearance) of trametinib in paediatric patients were comparable to those of adults. Weight was found to influence trametinib oral clearance, while age did not. The pharmacokinetic exposures of trametinib at the recommended weight-adjusted dose in paediatric patients were within range of those observed in adults.
Absorption
The trametinib oral solution was rapidly absorbed with a median time to achieve peak plasma concentration (Tmax) of 1 hour post-dose. The mean absolute oral bioavailability of the trametinib tablets was 72%. In a relative bioavailability study comparing the oral solution formulation and the tablet formulation after single-dose administration in the fasted state in adults, administration of the oral solution formulation resulted in a 12%, 10% and 71% higher AUC(0-inf), AUC(0-last) and Cmax respectively as compared to the tablet formulation.
Trametinib exposure increased in a dose-proportional manner between 0.125 mg and 4 mg following repeat once-daily dosing.
In the pivotal paediatric study, steady-state geometric mean (CV) Cmax and AUCtau were 22.7 ng/ml (41.1) and 339 ng*hr/ml (22.2%) in the LGG cohort and 21.3 ng/ml (36.3%) and 307 ng*hr/ml (22.8%) in the HGG cohort.
Trametinib accumulates with repeat daily dosing. A mean accumulation ratio of 6.0 was observed for the tablet formulation at 2 mg once-daily dose. Steady state was achieved by Day 15.
Food effect
The impact of food on the pharmacokinetics of the reconstituted oral solution has not been investigated. Administration of a single dose of trametinib (tablet formulation) with a high-fat, highcalorie meal resulted in a 70% and 10% decrease in Cmax and AUC, respectively, compared to fasted conditions.
Distribution
Binding of trametinib to human plasma proteins is 97.4%. Trametinib has a volume of distribution of approximately 1200 L determined following administration of a 5 g intravenous microdose.
Biotransformation
In vitro and in vivo studies demonstrated that trametinib is metabolised predominantly via deacetylation alone or in combination with mono-oxygenation. The deacetylated metabolite was further metabolised by glucuronidation. CYP3A4 oxidation is considered a minor pathway of metabolism. The deacetylation is mediated by the carboxyl-esterases 1b, 1c and 2, with possible contributions by other hydrolytic enzymes.
Following single and repeated doses of trametinib, trametinib as parent is the main circulating component in plasma.
Elimination
Mean terminal half-life of trametinib is 127 hours (5.3 days) after single-dose administration. The apparent clearance of trametinib in paediatric patients (median body weight: 32.85 kg) was 3.44 L/h (CV of 20%).
Total dose recovery was low after a 10-day collection period (<50%) following administration of a single oral dose of radiolabelled trametinib as a solution, due to the long elimination half-life. Trametinib-related material was excreted predominantly in the faeces (>80% of recovered radioactivity) and to a minor extent in urine (≤19%). Less than 0.1% of the excreted dose was recovered as parent in urine.
Medicinal product interactions
Effects of trametinib on drug-metabolising enzymes and transporters
In vitro and in vivo data suggest that trametinib is unlikely to affect the pharmacokinetics of other medicinal products. Based on in vitro studies, trametinib is not an inhibitor of CYP1A2, CYP2A6, CYP2B6, CYP2D6 and CYP3A4. Based on in vitro studies, trametinib is an inhibitor of CYP2C8, CYP2C9 and CYP2C19, an inducer of CYP3A4 and an inhibitor of the transporters OAT1, OAT3, OCT2, MATE1, OATP1B1, OATP1B3, P-gp and BCRP. However, based on the low dose and low clinical systemic exposure relative to the in vitro potency of inhibition or induction values, trametinib is not considered to be an in vivo inhibitor or inducer of these enzymes or transporters, although transient inhibition of BCRP substrates in the gut may occur (see section 4.5).
Effects of other medicinal products on trametinib
In vivo and in vitro data suggest that the pharmacokinetics of trametinib are unlikely to be affected by other medicinal products. Trametinib is not a substrate of CYP enzymes or of the transporters BCRP, OATP1B1, OATP1B3, OATP2B1, OCT1, MRP2 and MATE1. Trametinib is an in vitro substrate of BSEP and the efflux transporter P-gp. Although trametinib exposure is unlikely to be affected by inhibition of BSEP, increased levels of trametinib upon strong inhibition of hepatic P-gp cannot be excluded (see section 4.5).
Effects of trametinib on other medicinal products
The effect of repeat-dose trametinib on the steady-state pharmacokinetics of combination oral contraceptives, norethindrone and ethinyl estradiol, was assessed in a clinical study that consisted of 19 female patients with solid tumours. Norethindrone exposure increased by 20% and ethinyl estradiol exposure was similar when co-administered with trametinib. Based on these results, no loss of efficacy of hormonal contraceptives is expected when co-administered with trametinib.
Special patient populations
Hepatic impairment
Population pharmacokinetic analyses and data from a clinical pharmacology study in adult patients with normal hepatic function or with mild, moderate or severe bilirubin and/or AST elevations (based on National Cancer Institute [NCI] classification) indicate that hepatic function does not significantly affect trametinib oral clearance.
Renal impairment
Renal impairment is unlikely to have a clinically relevant effect on trametinib pharmacokinetics given the low renal excretion of trametinib. The pharmacokinetics of trametinib were characterised in 223 adult patients enrolled in clinical studies with trametinib who had mild renal impairment and 35 adult patients with moderate renal impairment using a population pharmacokinetic analysis. Mild and moderate renal impairment had no effect on trametinib exposure (<6% for either group). No data are available in patients with severe renal impairment (see section 4.2).
Race
There are insufficient data to evaluate the potential effect of race on trametinib pharmacokinetics as clinical experience is limited to Caucasians.
Gender
Based on population pharmacokinetic analyses in adult and paedatric patients, gender was found to influence trametinib oral clearance. Although female patients are predicted to have higher exposure than male patients, these differences are unlikely to be clinically relevant and no dose adjustment is warranted.
5.3. Preclinical safety data
Carcinogenicity studies with trametinib have not been conducted. Trametinib was not genotoxic in studies evaluating reverse mutations in bacteria, chromosomal aberrations in mammalian cells and micronuclei in the bone marrow of rats.
Trametinib may impair female fertility in humans, as in repeat-dose studies, increases in cystic follicles and decreases in corpora lutea were observed in female rats at exposures below the human clinical exposure based on AUC.
Additionally, in juvenile rats given trametinib, decreased ovarian weights, slight delays in hallmarks of female sexual maturation (vaginal opening and increased incidence of prominent terminal end buds within the mammary gland) and slight hypertrophy of the surface epithelium of the uterus were observed. All of these effects were reversible following an off-treatment period and attributable to pharmacology. However, in rat and dog toxicity studies up to 13 weeks in duration, there were no treatment effects observed in male reproductive tissues.
In embryo-foetal developmental toxicity studies in rats and rabbits, trametinib induced maternal and developmental toxicity. In rats, decreased foetal weights and increased post-implantation loss were seen at exposures below or slightly above the human clinical exposure based on AUC. In an embryofoetal developmental toxicity study with rabbits, decreased foetal body weight, increased abortions, increased incidence of incomplete ossification and skeletal malformations were seen at sub-clinical exposures based on AUC.
In repeat-dose studies the effects seen after trametinib exposure are found mainly in the skin, gastrointestinal tract, haematological system, bone and liver. Most of the findings are reversible after drug-free recovery. In rats, hepatocellular necrosis and transaminase elevations were seen after 8 weeks at ≥0.062 mg/kg/day (approximately 0.8 times human clinical exposure based on AUC).
In mice, lower heart rate, heart weight and left ventricular function were observed without cardiac histopathology after 3 weeks at ≥0.25 mg/kg/day trametinib (approximately 3 times human clinical exposure based on AUC) for up to 3 weeks. In adult rats, mineralisation of multiple organs was associated with increased serum phosphorus and was closely associated with necrosis in heart, liver and kidney and haemorrhage in the lung at exposures comparable to the human clinical exposure. In rats, hypertrophy of the physis and increased bone turnover were observed. In rats and dogs given trametinib at or below human clinical exposures, bone marrow necrosis, lymphoid atrophy in thymus and GALT and lymphoid necrosis in lymph nodes, spleen and thymus were observed, which have the potential to impair immune function. In juvenile rats, increased heart weight with no histopathology was observed at 0.35 mg/kg/day (approximately 2 times the human clinical exposure based on AUC).
Trametinib was phototoxic in an in vitro mouse fibroblast 3T3 Neutral Red Uptake (NRU) assay at significantly higher concentrations than clinical exposures (IC50 at 2.92 µg/ml, ≥130 times the human clinical exposure based on Cmax), indicating that there is low risk for phototoxicity to patients taking trametinib.
Combination with dabrafenib
In a study in dogs in which trametinib and dabrafenib were given in combination for 4 weeks, signs of gastrointestinal toxicity and decreased lymphoid cellularity of the thymus were observed at lower exposures than in dogs given trametinib alone. Otherwise, similar toxicities were observed as in comparable monotherapy studies.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.