SPIOLTO RESPIMAT Inhalation solution Ref.[51419] Active ingredients: Olodaterol Olodaterol and Tiotropium bromide Tiotropium
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2022 Publisher: Boehringer Ingelheim International GmbH, Binger Strasse 173, 55216 Ingelheim am Rhein, Germany
4.1. Therapeutic indications
Spiolto Respimat is indicated as a maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD).
4.2. Posology and method of administration
Posology
The medicinal product is intended for inhalation use only. The cartridge can only be inserted and used in the Respimat inhaler.
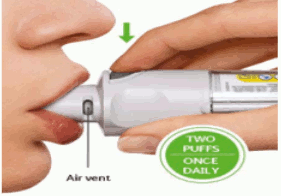
Two puffs from the Respimat inhaler comprise one medicinal dose.
Adults
The recommended dose is 5 microgram tiotropium and 5 microgram olodaterol given as two puffs from the Respimat inhaler once daily, at the same time of the day.
The recommended dose should not be exceeded.
Elderly population
Elderly patients can use Spiolto Respimat at the recommended dose.
Hepatic impairment and Renal impairment
Spiolto Respimat contains tiotropium which is a predominantly renally excreted drug and olodaterol, which is predominantly metabolized in the liver.
Hepatic impairment
Patients with mild and moderate hepatic impairment can use Spiolto Respimat at the recommended dose.
There are no data available for use of olodaterol in patients with severe hepatic impairment.
Renal impairment
Renally impaired patients can use Spiolto Respimat at the recommended dose.
For patients with moderate to severe impairment (creatinine clearance ≤ 50 ml/min) see 4.4 and 5.2.
Spiolto Respimat contains olodaterol. There is limited experience with the use of olodaterol in patients with severe renal impairment.
Paediatric population
There is no relevant use of Spiolto Respimat in the paediatric population (under 18 years).
Method of administration
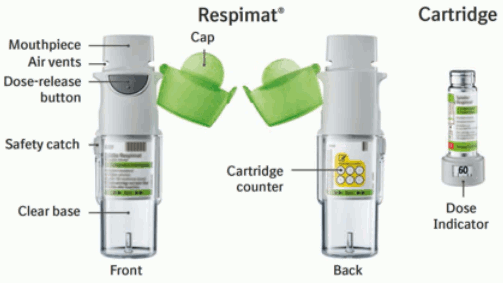
This medicinal product is intended for inhalation use only. The cartridge can only be inserted and used in the Respimat re-usable inhaler. Respimat is an inhaler device that generates a spray for inhalation. It is meant for use by a single patient and intended for multiple doses delivered by one cartridge.
The Respimat re-usable inhaler allows for replacement of the cartridge, and can be used with up to 6 cartridges.
Patients should read the instructions on how to use the Respimat re-usable inhaler before they start using Spiolto Respimat.
To ensure proper administration of the medicinal product, the patient should be shown how to use the inhaler by a physician or other health care professional.
Instructions for handling and use of the Respimat re-usable inhaler
The patient will need to use this inhaler only ONCE A DAY. Each time used take TWO PUFFS.
- If Spiolto Respimat has not been used for more than 7 days release one puff towards the ground.
- If Spiolto Respimat has not been used for more than 21 days repeat steps 4 to 6 under ‘Prepare for use’ until a cloud is visible. Then repeat steps 4 to 6 three more times.
How to care for the Respimat re-usable inhaler
Clean the mouthpiece including the metal part inside the mouthpiece with a damp cloth or tissue only, at least once a week.
Any minor discoloration in the mouthpiece does not affect the Respimat re-usable inhaler performance.
If necessary, wipe the outside of the Respimat re-usable inhaler with a damp cloth.
When to replace the inhaler
When the patient has used an inhaler with 6 cartridges, get a new Spiolto Respimat pack containing an inhaler.
Prepare for use
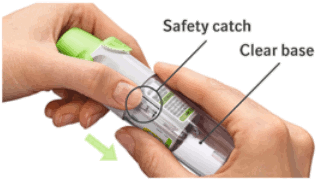
1. Remove clear base
- Keep the cap closed.
- Press the safety catch while pulling off the clear base with the other hand.
2. Insert cartridge
- Insert the cartridge into the inhaler.
- Place the inhaler on a firm surface and push down firmly until it clicks into place.
3. Track cartridge and put the clear base back
- Mark the check-box on inhaler’s label to track the number of cartridges.
- Put the clear base back into place until it clicks.
4. Turn
- Keep the cap closed.
- Turn the clear base in the direction of the arrows on the label until it clicks (half a turn).
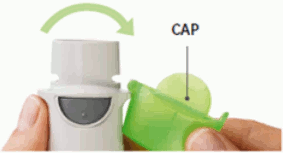
5. Open
- Open the cap until it snaps fully open.
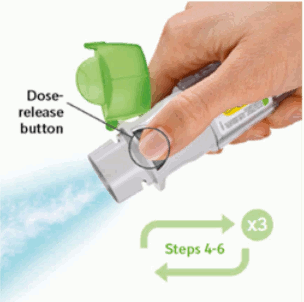
6. Press
- Point the inhaler toward the ground.
- Press the dose-release button.
- Close the cap.
- Repeat steps 4-6 until a cloud is visible.
- After a cloud is visible, repeat steps 4-6 three more times.
The inhaler is now ready to use and will deliver 60 puffs (30 doses).
TURN
- Keep the cap closed.
- TURN the clear base in the direction of the arrows on the label until it clicks (half a turn).
OPEN
- OPEN the cap until it snaps fully open.
PRESS
- Breathe out slowly and fully.
- Close the lips around the mouthpiece without covering the air vents. Point the Inhaler to the back of the throat.
- While taking a slow, deep breath through the mouth, PRESS the dose-release button and continue to breathe in slowly for as long as comfortable.
- Hold the breath for 10 seconds or for as long as comfortable.
- Repeat TURN, OPEN, PRESS for a total of 2 puffs.
- Close the cap until the inhaler is used again.
When to replace the Spiolto Respimat cartridge
The dose indicator shows how many puffs remain in the cartridge.
60 puffs remaining.
Less than 10 puffs remaining. Obtain a new cartridge.
The cartridge is used up. Turn the clear base to loosen it. The inhaler is now in a locked position. Pull off the cartridge from the inhaler. Insert a new cartridge until it clicks (refer to step 2). The new cartridge will stick out more than the very first cartridge (continue with step 3). Remember to put the clear base back to unlock the inhaler.
4.9. Overdose
There is limited information on overdosing with Spiolto Respimat. Spiolto Respimat has been studied up to 5 microgram/10 microgram (tiotropium/olodaterol) in COPD patients and up to 10 microgram/40 microgram (tiotropium/olodaterol) in healthy subjects; no clinically relevant effects were observed. An overdose could lead to exaggerated anti-muscarinic effects of tiotropium and/or exaggerated β2 agonists effects of olodaterol.
Symptoms
Overdose of anticholinergic tiotropium
High doses of tiotropium may lead to anticholinergic signs and symptoms. However, there were no systemic anticholinergic adverse effects following a single inhaled dose of up to 340 microgram tiotropium bromide in healthy volunteers. Additionally, no relevant adverse events, beyond dry mouth/throat and dry nasal mucosa were observed following 14-day dosing of up to 40 microgram tiotropium inhalation solution in healthy volunteers with the exception of pronounced reduction in salivary flow from day 7 onwards.
Overdose of β2-agonist olodaterol
An overdose of olodaterol is likely to lead to exaggerated effects typical of beta2-adrenergic agonists, e.g. myocardial ischaemia, hypertension or hypotension, tachycardia, arrhythmias, palpitation, dizziness, nervousness, insomnia, anxiety, headache, tremor, dry mouth, muscle spasms, nausea, fatigue, malaise, hypokalemia, hyperglycemia, and metabolic acidosis.
Treatment of overdose
Treatment with Spiolto Respimat should be discontinued. Supportive and symptomatic treatment is indicated. Serious cases should be hospitalised. Use of cardioselective beta-blockers may be considered, but only subject to extreme caution since the use of beta-adrenergic blocker medication may provoke bronchospasm.
6.3. Shelf life
3 years.
In-use shelf life of the cartridge: 3 months.
In-use shelf life of the inhaler: 1 year.
Recommended use: 6 cartridges per inhaler.
Note: The functioning of the RESPIMAT re-usable inhaler has been demonstrated in tests for 540 actuations (corresponding to 9 cartridges).
6.4. Special precautions for storage
Do not freeze.
6.5. Nature and contents of container
Type and material of the container in contact with the medicinal product:
Solution filled into a polyethylene/polypropylene cartridge with a polypropylene cap with integrated silicone sealing ring. The cartridge is enclosed within an aluminium cylinder.
Each cartridge contains 4 ml inhalation solution.
Pack sizes and devices supplied:
Single pack: 1 Respimat re-usable inhaler and 1 cartridge, providing 60 puffs (30 medicinal doses).
Triple pack: 1 Respimat re-usable inhaler and 3 cartridges, providing 60 puffs (30 medicinal doses) each.
Single refill pack: 1 cartridge, providing 60 puffs (30 medicinal doses).
Triple refill pack: 3 cartridges, providing 60 puffs (30 medicinal doses) each.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.