SPIRIVA Inhalation powder, hard capsule Ref.[7493] Active ingredients: Tiotropium
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2023 Publisher: Boehringer Ingelheim International GmbH, Binger Straße 173, D-55216 Ingelheim am Rhein, Germany
Therapeutic indications
Spiriva is indicated as a maintenance bronchodilator treatment to relieve symptoms of patients with chronic obstructive pulmonary disease (COPD).
Posology and method of administration
Posology
The medicinal product is intended for inhalation use only.
The recommended dosage of tiotropium bromide is inhalation of the contents of one capsule once daily with the HandiHaler device at the same time of day.
The recommended dose should not be exceeded.
Spiriva capsules are only for inhalation and not for oral intake.
Spiriva capsules must not be swallowed.
Spiriva capsules should only be inhaled with the HandiHaler device.
Special populations
Geriatric patients can use tiotropium bromide at the recommended dose.
Renally impaired patients can use tiotropium bromide at the recommended dose. For patients with moderate to severe impairment (creatinine clearance ≤50 ml/min) see section 4.4 and section 5.2.
Hepatically impaired patients can use tiotropium bromide at the recommended dose (see section 5.2).
Paediatric population
COPD
There is no relevant use in the paediatric population (below 18 years) in the indication stated under section 4.1.
Cystic fibrosis
The safety and efficacy of Spiriva 18 microgram in children and adolescents has not been established. No data are available.
Method of administration
The HandiHaler is an inhalation device especially designed to enable patients to inhale the medication contained in Spiriva capsules.
The HandiHaler must not be used to take any other medication. It is a single patient device intended for multiple uses.
To ensure proper administration of the medicinal product the patient should be trained on how to use the inhaler by the physician or by other healthcare professional.
Instructions for handling and use
 | Remember to carefully follow your doctor's instructions for using Spiriva. After first use, you can use your HandiHaler for up to one year to take your medication. |
 | The HandiHaler 1 Dust cap 2 Mouthpiece 3 Base 4 Piercing button 5 Centre chamber |
 | 1. To release the dust cap press the piercing button completely in and let go. |
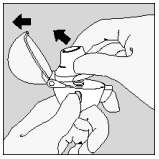 | 2. Open the dust cap completely by pulling it upwards. Then open the mouthpiece by pulling it upwards. |
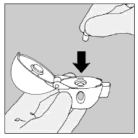 | 3. Remove a Spiriva capsule from the blister (only immediately before use, see blister handling) and place it in the centre chamber (5), as illustrated. It does not matter which way the capsule is placed in the chamber. |
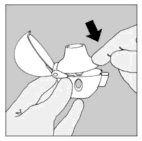 | 4. Close the mouthpiece firmly until you hear a click, leaving the dust cap open. |
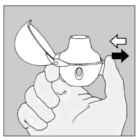 | 5. Hold the HandiHaler device with the mouthpiece upwards and press the piercing button completely in only once, and release. This makes holes in the capsule and allows the medication to be released when you breathe in. |
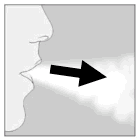 | 6. Breathe out completely. Important: Please avoid breathing into the mouthpiece at any time. |
 | 7. Raise the HandiHaler to your mouth and close your lips tightly around the mouthpiece. Keep your head in an upright position and breathe in slowly and deeply but at a rate sufficient to hear or feel the capsule vibrate. Breathe in until your lungs are full; then hold your breath as long as comfortable and at the same time take the HandiHaler out of your mouth. Resume normal breathing. Repeat steps 6 and 7 once, in order to empty the capsule completely. |
 | 8. Open the mouthpiece again. Tip out the used capsule and dispose. Close the mouthpiece and dust cap for storage of your HandiHaler device. |
Cleaning your HandiHaler
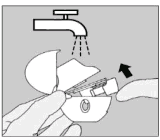 | Clean the HandiHaler once a month. Open the dust cap and mouthpiece. Then open the base by lifting the piercing button. Rinse the complete inhaler with warm water to remove any powder. Dry the HandiHaler thoroughly by tipping excess of water out on a paper towel and air-dry afterwards, leaving the dust cap, mouthpiece and base open. It takes 24 hours to air dry, so clean it immediately after use so that it will be ready for your next dose. If needed, the outside of the mouthpiece may be cleaned with a moist but not wet tissue. |
Blister handling
 | A. Separate the Spiriva blister strips by tearing along the perforation. |
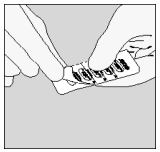 | B. Peel back foil (only immediately before use) using the tab until one capsule is fully visible. In case a second capsule is exposed to air inadvertently this capsule has to be discarded. |
 | C. Remove capsule. |
Spiriva capsules contain only a small amount of powder so that the capsule is only partially filled.
Overdose
High doses of tiotropium bromide may lead to anticholinergic signs and symptoms.
However, there were no systemic anticholinergic adverse effects following a single inhaled dose of up to 340 microgram tiotropium bromide in healthy volunteers. Additionally, no relevant adverse effects, beyond dry mouth, were observed following 7 day dosing of up to 170 microgram tiotropium bromide in healthy volunteers. In a multiple dose study in COPD patients with a maximum daily dose of 43 microgram tiotropium bromide over four weeks no significant undesirable effects have been observed.
Acute intoxication by inadvertent oral ingestion of tiotropium bromide capsules is unlikely due to low oral bioavailability.
Shelf life
2 years.
After first opening of the blister use within the next 9 days.
Discard the HandiHaler device 12 months after first use.
Special precautions for storage
Do not store above 25°C.
Do not freeze.
Nature and contents of container
Aluminium/PVC/Aluminium peel-off blister containing 10 capsules.
The HandiHaler is a single dose inhalation device made from acrylonitrile butadiene styrene (ABS) plastic materials and stainless steel. The capsule chamber is made from methyl-methacrylate-acrylonitrile-butadiene-styrene (MABS) or polycarbonate (PC) plastic material.
Package sizes and devices supplied:
- Cardboard box containing 30 capsules (3 blisters).
- Cardboard box containing 60 capsules (6 blisters).
- Cardboard box containing 90 capsules (9 blisters).
- Cardboard box containing HandiHaler device and 10 capsules (1 blister).
- Cardboard box containing HandiHaler device and 30 capsules (3 blisters).
- Hospital pack: Bundle pack containing 5 cardboard boxes of 30 capsules plus HandiHaler device.
- Hospital pack: Bundle pack containing 5 cardboard boxes of 60 capsules.
The HandiHaler device is packed/available in a cardboard box.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.