SPORANOX I.V. Concentrate and solvent for solution for infusion Ref.[6947] Active ingredients: Itraconazole
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2017 Publisher: Janssen-Cilag Ltd., 50-100 Holmers Farm Way, High Wycombe, Buckinghamshire, HP12 4EG, UK
Therapeutic indications
Sporanox IV is indicated for the treatment of histoplasmosis.
Sporanox IV is indicated in the following systemic fungal conditions when first-line systemic anti-fungal therapy is inappropriate or has proved ineffective. (This may be due to underlying pathology, insensitivity of the pathogen or drug toxicity).
Treatment of aspergillosis, candidosis and cryptococcosis (including cryptococcal meningitis): in immunocompromised patients with cryptococcosis and in all patients with cryptococcosis of the central nervous system.
Consideration should be given to national and/or local guidance regarding the appropriate use of antifungal agents.
Posology and method of administration
This product is supplied with an extension line with a 2-way stopcock and 0.2 μm in-line filter. The dedicated extension line including the in-line filter must be used to ensure the correct administration of the product (see section 6.6).
Sporanox IV is given on the first two days in a loading dose twice daily, followed by once daily dosing.
Day 1 and 2 of the treatment: 1-hour infusion of 200 mg (60 ml of the admixed solution) Sporanox IV twice daily (see section 6.6).
From day 3 on: one 1-hour infusion of 200 mg (60 ml of the admixed solution) Sporanox IV each day. Safety for periods longer than 14 days has not been established.
Use in children
Clinical data on the use of Sporanox IV in paediatric patients are limited. The use of Sporanox IV in paediatric patients is not recommended unless it is determined that the potential benefit outweighs the potential risks (see section 4.4).
Use in elderly
Since clinical data of the use of Sporanox IV in elderly patients are limited, it is advised to use Sporanox IV in these patients only if the potential benefit outweighs the potential risks. In general, it is recommended that the dose selection for an elderly patient should be taken into consideration, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy (see section 4.4).
Use in patients with renal impairment
Limited data are available on the use of intravenous itraconazole in patients with renal impairment.
Hydroxypropyl-β-cyclodextrin, a required component of Sporanox intravenous formulation, is eliminated through glomerular filtration. Therefore, in patients with severe renal impairment defined as creatinine clearance below 30 ml/min the use of Sporanox IV is contraindicated (see section 4.3).
In patients with mild and moderate renal impairment, Sporanox IV should be used with caution. Serum creatinine levels should be closely monitored and, if renal toxicity is suspected, consideration should be given to changing to the oral capsule formulation (see sections 4.4 and 5.2).
Use in patients with hepatic impairment
Limited data are available on the use of itraconazole in patients with hepatic impairment. Caution should be exercised when this drug is administered in this patient population (see section 5.2).
Overdose
Symptoms
In general, adverse events reported with overdose have been consistent with adverse drug reactions already listed in this SmPC for itraconazole (see section 4.8).
Treatment
In the event of overdose, supportive measures should be employed. Itraconazole cannot be removed by haemodialysis. No specific antidote is available.
Shelf life
Shelf life
Sporanox IV (as packaged): 2 years.
0.9% Sodium Chloride Injection: 3 years.
Admixed Solution: 24 hours.
Special precautions for storage
Sporanox IV: Do not store above 25°C. Store in the original container.
0.9% Sodium Chloride Injection: Do not store above 25°C. Do not freeze.
Admixed solution: Protect from direct sunlight.
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless the dilution of the admixture has taken place in controlled and validated aseptic conditions.
Nature and contents of container
Sporanox IV: 25 ml siliconised type I colourless glass ampoule with 25 ml containing 250 mg itraconazole.
0.9% Sodium Chloride: Flexible polypropylene infusion bag, equipped with a flexible inlet and outlet port, and containing 52 to 56 ml of 0.9% Sodium Chloride Injection.
Extension line: Polyvinylchloride tubing with 2-way stopcock and in-line filter.
Special precautions for disposal and other handling
Itraconazole has the potential to precipitate when 25 ml of Sporanox IV concentrate are diluted in solutions other than 50 ml 0.9% Sodium Chloride Injection. The full amount of 25 ml of Sporanox IV concentrate from the ampoule must be diluted into the Sodium Chloride Infusion Bag, which is intended to be used exclusively in combination with Sporanox IV concentrate. Only the components of unit sales pack (e.g. saline bag, an extension line with a 2-way stopcock and 0.2 μm in-line filter, and Sporanox IV ampoule) must be used. Sporanox IV cannot be co-administered with other drugs or fluids (see section 6.2).
Prior to starting the admixing process, the Sporanox IV concentrate, and the solvent (Sodium Chloride) must be visually inspected. Only clear solutions free from foreign particles should be used for the preparation of the admixture.
The full amount of Sporanox IV concentrate must be injected into the Sodium Chloride bag in a slow single action (up to 60 seconds). During the admixing process opalescence may appear but will clear after gently mixing. When visually inspecting the bag after admixing and prior to administration, product intrinsic aggregates may be observed. These aggregates do not affect the quality of the product. The dedicated extension line with the 0.2 μm in-line filter must be used to prevent aggregates from reaching the recipient’s circulation.
Sporanox IV should be prepared for administration according to the following instructions:
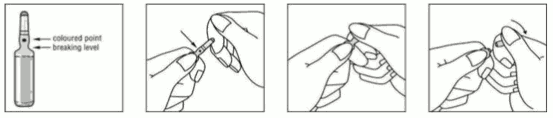
Opening ampoule:
Break the ampoule as shown:
Opening sodium chloride bag:
Tear outer wrap at notch and remove infusion bag.
Flush procedure before the infusion
Before the infusion, the catheter should be flushed to avoid compatibility problems between residual amounts of other drugs and itraconazole.
- Fill the extension line provided with the kit containing the 0.2 μm in-line filter with sterile 0.9% sodium chloride solution and connect directly to the indwelling intravenous catheter.
- Flush the extension line provided with the kit and indwelling intravenous catheter with sterile 0.9% sodium chloride solution.
Admixing Sporanox IV Concentrate and 0.9% Sodium Chloride Injection
- Each component must be at room temperature.
- Admix only in the infusion bag provided. Using aseptic technique and an additive delivery needle of appropriate length (not supplied with the kit), draw up all concentrate from the ampoule and subsequently add the Sporanox IV concentrate to the infusion bag by puncturing the resealable additive port and inject.
- Add the entire volume (25 ml) of Sporanox IV concentrate to the bag in a slow single action (up to 60 seconds). During the admixing process some opalescence may appear. This is a normal phenomenon for the product and will disappear after the full content of the 25 ml of Sporanox IV has been diluted into the Sodium Chloride infusion bag and after gentle mixing. Withdraw needle after injecting the Sporanox IV concentrate into the bag.
- Gently mix the content of the bag once the Sporanox IV concentrate is completely transferred to the bag. The admixture will become clear but product intrinsic aggregates (described as fibrous to flake-like, non-crystalline, white particles) may be observed. These aggregates do not affect the quality of the product.
- The admixture should be used immediately and should be protected from direct sunlight. During administration, exposure to normal room light is acceptable (see sections 6.3 and 6.4).
Infusion:
- The admixed solution is intended for single-dose infusion only. No administration should occur if the solution is a milky white colour that does not disappear after gentle mixing, or contains foreign matter, or if the infusion bag is damaged. The infusion bag should now contain 25 ml Sporanox IV concentrate and 50 ml 0.9% Sodium Chloride Injection.
- Note: An infusion line with drip chamber is not supplied with the kit. Close the flow control device (e.g. rotary clamp) on the infusion line. Remove the breakable part of the outlet port. Using aseptic technique, push the pin of the infusion line in the flexible port of the infusion bag.
- Slowly release the flow control device and fill the drip chamber to half full by squeezing (pumping) it.
- Open the flow control device until all air has been expelled from the infusion line.
- Connect the infusion line to the two-way stopcock of the extension line.
- The Sporanox infusion is now ready for intravenous infusion to the patient.
- Adjust the infusion rate to 1 ml/min (approximately 25 drops/min) by means of a flow control device (e.g. rotary clamp or infusion pump).
- Administer 60 ml of the solution to the patient over approximately one hour.
- Stop the infusion when 60 ml is administered.
- Note that 200 mg of itraconazole has been administered.
- Flush the line as per the flushing procedure described below.
Flush procedure after the infusion:
After the infusion a complete flush procedure must be started to clean the catheter. This is done to avoid compatibility problems between residual amounts of itraconazole and other drugs which later could be administered through the same catheter.
- Flush the extension line and catheter with 15–20 ml of sterile 0.9% sodium chloride solution at the level of the 2-way stop cock, just before the 0.2 μm in-line filter.
- Perform the flush in a continuous run of 30 seconds to 15 minutes.
- After flushing, disconnect and discard the bag, the infusion line and the extension line.
- Do not re-sterilise or re-use the Sporanox infusion set.
- To avoid precipitation other medication should only be administered via the catheter after flushing.
- If using a multi-lumen catheter, other medication may not be administered until the Sporanox IV infusion has been completed and the catheter has been flushed.
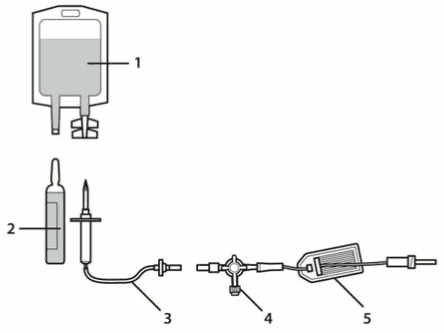
1. Sodium chloride infusion bag
2. Sporanox IV ampoule
3. Infusion line with drip chamber (not provided)
4. & 5. Extension line with 2-way stopcock and in-line filter
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.