TAMOXIFEN Tablet Ref.[7488] Active ingredients: Tamoxifen
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2018 Publisher: Generics [UK] Ltd t/a Mylan, Station Close, Potters Bar, Herts, EN6 1TL
Pharmacodynamic properties
Pharmacotherapeutic group: Hormone antagonists and related agents, anti-oestrogens
ATC Code: L02BA01
Mechanism of action
Tamoxifen citrate is an oestrogen antagonist which is believed to compete with oestrogen for binding sites in target organs. It does not have androgenic properties.
It is used as an alternative to androgens and oestrogens in the management of breast cancer in doses equivalent to 10 and 20mg of Tamoxifen twice daily by mouth.
Tamoxifen is a non-steroidal, triphenylethylene-based drug which displays a complex spectrum of oestrogen antagonist and oestrogen agonist-like pharmacological effects in different tissues. In breast cancer patients, at the tumour level, tamoxifen acts primarily as an antioestrogen, preventing oestrogen binding to the oestrogen receptor. However, clinical studies have shown some benefit in oestrogen receptor negative tumours which may indicate other mechanisms of action. In the clinical situation, it is recognised that tamoxifen leads to reductions in levels of blood total cholesterol and low density lipoproteins in postmenopausal women of the order of 10–20%. Tamoxifen does not adversely affect bone mineral density.
Paediatric population
An uncontrolled trial was undertaken in a heterogenous group of 28 girls aged 2 to 10 years with McCune Albright Syndrome (MAS), who received 20 mg once a day for up to 12 months duration. Among the patients who reported vaginal bleeding during the pre-study period, 62% (13 out of 21 patients) reported no bleeding for a 6-month period and 33% (7 out of 21 patients) reported no vaginal bleeding for the duration of the trial. Mean uterine volume increased after 6 months of treatment and doubled at the end of the one-year study. While this finding is in line with the pharmacodynamic properties of tamoxifen, a causal relationship has not been established (see section 4.4). There are no long-term safety data in children. In particular, the long-term effects of tamoxifen on growth, puberty and general development have not been studied.
CYP2D6 polymorphism
CYP2D6 polymorphism status may be associated with variability in clinical response to tamoxifen. The poor metaboliser status may be associated with reduced response. The consequences of the findings for the treatment of CYP2D6 poor metabolisers have not been fully elucidated (see sections 4.4, 4.5 and 5.2).
CYP2D6 genotype
Available clinical data suggest that patients, who are homozygote for non-functional CYP2D6 alleles, may experience reduced effect of tamoxifen in the treatment of breast cancer.
The available studies have mainly been performed in postmenopausal women (see sections 4.4 and 5.2).
Primary reduction of breast cancer risk
Tamoxifen reduces, but does not eliminate the risk of breast cancer. In clinical trials, Tamoxifen decreased the incidence of oestrogen receptor-positive tumours, but did not alter the incidence of oestrogen receptor-negative tumours. The use of Tamoxifen should be as part of a program including regular breast surveillance tailored to the individual woman, taking into account her risk of breast cancer.
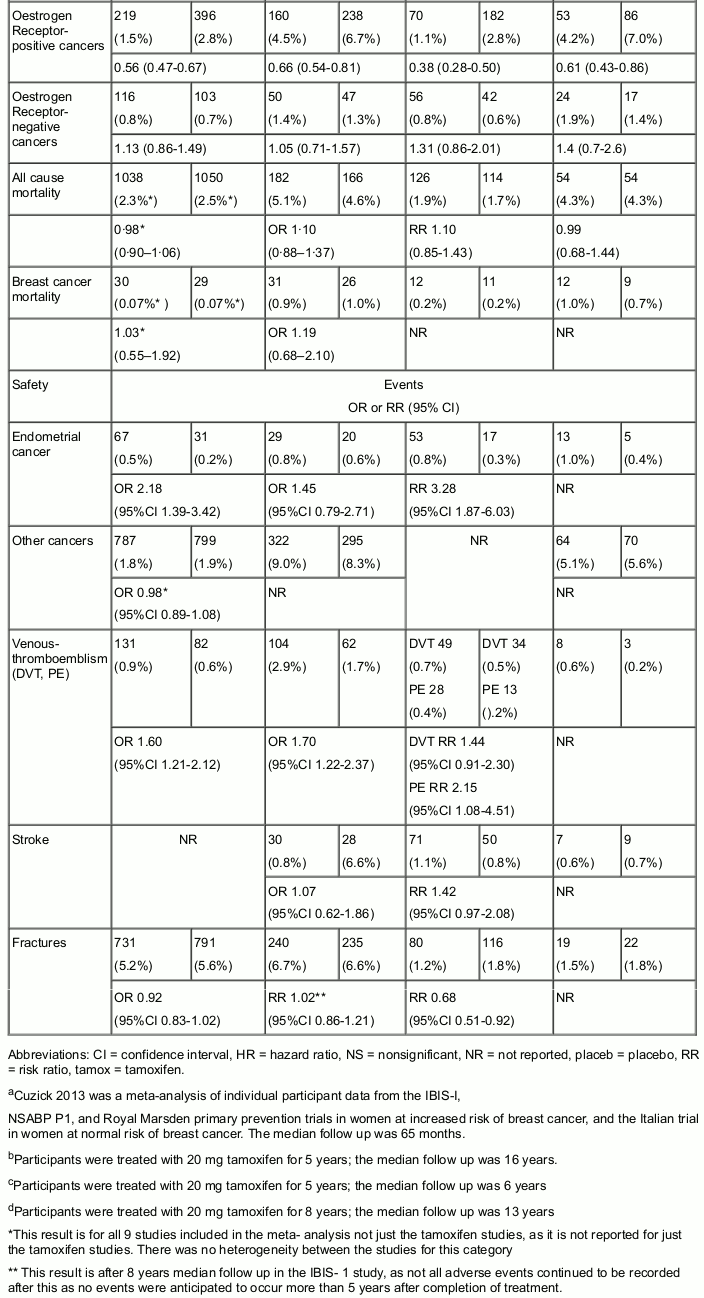
The breast cancer primary risk reduction trials include the International Breast Cancer Intervention Study (IBIS-1), the National Surgical Adjuvant Breast and Bowel Project PI study (NSABP P1), and the Royal Marsden Hospital chemoprevention trial (Royal Marsden). All trials were double-blind placebo controlled randomised trials of oral tamoxifen (20 mg per day) for the primary reduction of breast cancer risk in women at increased risk of breast cancer. Women were treated for 5 years (IBIS-1 and NSABP P1) or 8 years (Royal Marsden) and followed for up to 20 years.
The IBIS-1, NSABP PI, and Royal Marsden trials all defined breast cancer risk differently, and recruited women with both moderate or high lifetime risk: IBIS-1 included women with a two-fold relative risk if they were aged 45 to 70 years, a fourfold relative risk if they were aged 40 to 44 years, or a ten-fold relative risk if they were aged 35 to 39 years; NSABP P1 included women aged ≥60 years or aged 35 to 59 years with a 5-year predicted risk for breast cancer of at least 1.66% as determined using a modified Gail’s model or a history of Lobular Carcinoma In Situ (LCIS) or atypical hyperplasia; and Royal Marsden included healthy women aged 30 to 70 years old with an increased risk of developing breast cancer based on family history.
All trials excluded women with breast cancer (apart from Lobular Carcinoma In Situ – LCIS), a history of invasive cancer, pregnancy, and current or past deep vein thrombosis or pulmonary embolism. Other relevant exclusion criteria included the current use of oral contraceptives (NSABP P1, Royal Marsden), recent or current hormone replacement therapy (NSABP P1), and current anticoagulant use (IBIS-1).
The majority of women in all trials were aged 59 years or below. NSABP PI included the largest proportion of women aged 60 years or over (30%). In NSABP P1, the majority of women were white (96%); race was not reported in the other trials. A substantial proportion of women in all trials were premenopausa1 (46% in IBIS-1 and 65% in Royal Marsden) or younger than 50 years old (37% NSABP P1).
A summary of the key entry criteria for each of the trials are shown in Table 2.
Table 2. Summary of the Key Criteria Used to Select Patients in Each of the Main Studies:
| Study | Key Entry Criteria | |
|---|---|---|
| IBIS 1 | Aged 35-70 years | |
| No previous invasive cancer (except non-melanoma skin cancer) | ||
| Relative risk of developing breast cancer: | ||
| * At least two-fold in women aged 45-70 | ||
| * At least four-fold in women aged 40-44 | ||
| * At least ten-fold in women aged 35-39 | ||
| Calculated using a specifically designed model based on family history and standard risk factors | ||
| NSABP P1 | Aged >35 years | |
| No clinical evidence of breast cancer | ||
| 5-year predicted risk >1.66% of developing breast cancer based on the Gail model, or a history of LCIS or atypical hyperplasia based on a multivariable logistic regression model | ||
| STAR | Aged >35 years | 5 yr predicted risk of >1.66% of developing breast cancer based on Gail model |
| Aged 30 – 70 years old | ||
| No clinical evidence of breast cancer | ||
| Increased risk of developing breast cancer based on family history. |
Efficacy results from the trials are shown in Table 3, which includes results of a metaanalysis of individual participant data from over 28,000 women who were treated with tamoxifen or placebo for the primary reduction of breast cancer risk. The results of the individual trials were generally consistent with the findings in the metaanalysis and the risk reduction effects of tamoxifen lasted for more than 10 years after treatment ended.
Table 3. Summary of Key Efficacy and Safety Results from the Primary Risk Reduction Trials:
Mortality was a secondary outcome measure for the IBIS-1, NSABP P1 and Royal Marsden trials. In comparing the tamoxifen and placebo arms, no significant difference was found for mortality in each trial. This outcome may be due to confounding factors in these trials such as low event rates, underpowering, close screening leading to early detection of events and subsequent breast cancer treatments.
Concomitant use of Hormone Replacement Therapy
The IBIS-1 trial found that tamoxifen was effective in reducing the risk of breast cancer in women who were not taking hormone replacement therapy. For women who did use hormone replacement therapy, there was no significant reduction in the risk of developing invasive breast cancers: 110 vs 124 (HR 0.88, 95% CI 0.68-1.13, p=0.31). These findings were consistent over the 20-year study period. In the NSABP P1 trial, women who were taking hormone replacement therapy were excluded from the trial. The Royal Marsden trial was not powered to demonstrate an effect. Therefore, the concomitant use of tamoxifen and hormone replacement therapy is not recommended for primary prevention of breast cancer.
Effects of age and menopausal status
No age-related effects of tamoxifen on breast cancer incidence were reported in the primary risk reduction trials. Analyses according to age were performed in the final analyses of the IBIS-1 and the NSABP P1 trials. In the IBIS-1 trial, breast cancer incidence was significantly decreased in the tamoxifen vs the placebo group in women aged ≤50 years and >50 years, In the NSABP P1 trial, invasive breast cancer incidence was significantly decreased in the tamoxifen vs the placebo group in women aged ≤49 years, 50 to 59 years, and ≥60 years. Thus, no age-related effects of tamoxifen on breast cancer incidence were reported in the trials.
Analyses according to menopausal status were performed in the 96-month analysis of the IBIS-1 trial. In the IBIS-1 trial, tamoxifen significantly reduced the risk of breast cancer in premenopausal women compared with placebo. It should be noted that the IBIS-1 trial was not sufficiently powered to detect a difference specifically in postmenopausal women. In the NSABP P1 trial, the incidence of invasive breast cancer was significantly lower in the tamoxifen vs placebo group in women aged ≥60 years, who would have been postmenopausal (40 vs 80, RR 0.49, 95% CI0.33-0.73).
Lobular carcinoma in situ and atypical hyperplasia
In NSABP P1, there was a 75% breast cancer risk reduction in women with a history of atypical hyperplasia compared with a 37% risk reduction in women with no history of atypical hyperplasia (RR 0.63, 95% CI 0.50-0.78). The risk reductions for women with and without lobular carcinoma in situ were similar.
Pharmacokinetic properties
Absorption
After oral administration, tamoxifen is absorbed rapidly with peak plasma concentrations of Tamoxifen occurring 4 to 7 hours after an oral dose. Steady state concentrations (about 300 ng/ml) are achieved after four weeks treatment with 40 mg daily.
Distribution
Tamoxifen is highly protein bound to serum albumin (>99%).
Biotransformation and elimination
Plasma clearance is reported to be biphasic and the terminal half-life may be longer than 7 days for tamoxifen itself, whereas that for N-desmethyltamoxifen, the principal circulating metabolite, is 14 days.
It is extensively metabolised by hydroxylation, demethylation and conjugation, the major serum metabolite being N-desmethyltamoxifen, and is excreted slowly in the faeces, mainly as conjugates. Small amounts are excreted in the urine. Tamoxifen appears to undergo enterohepatic circulation.
Paediatric population
In a clinical study where girls between 2 and 10 years with McCune Albright Syndrome (MAS) received 20 mg tamoxifen once a day for up to 12 months duration, there was an age-dependent decrease in clearance and an increase in exposure (AUC), (with values up to 50% higher in the youngest patients) compared with adults.
CYP2D6 polymorphism
Tamoxifen is metabolised mainly via CYP3A4 to N-desmethyl-tamoxifen, which is further metabolised by CYP2D6 to another active metabolite endoxifen. In patients who lack the enzyme CYP2D6 endoxifen concentrations are approximately 75% lower than in patients with normal CYP2D6 activity. Administration of strong CYP2D6 inhibitors reduces endoxifen circulating levels to a similar extent.
Preclinical safety data
Tamoxifen was not mutagenic in a range of in vitro and in vivo mutagenicity tests. Tamoxifen was genotoxic in some in vitro and in vivo genotoxicity tests in rodents. Gonadal tumours in mice and liver tumours in rats receiving tamoxifen have been reported in long-term studies. The clinical relevance of these findings has not been established.
Tamoxifen is a drug on which extensive clinical experience has been obtained.
Relevant information for the prescriber is provided elsewhere in the Summary of Product Characteristics.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.