TECENTRIQ Concentrate for solution for infusion Ref.[6321] Active ingredients: Atezolizumab
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Roche Registration GmbH, Emil-Barell-Strasse 1, 79639 Grenzach-Wyhlen, Germany
Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, monoclonal antibodies and antibody drug conjugates, PD-1/PDL-1 (Programmed cell death protein 1/death ligand 1) inhibitors
ATC code: L01FF05
Mechanism of action
Programmed death-ligand 1 (PD-L1) may be expressed on tumour cells and/or tumour-infiltrating immune cells, and can contribute to the inhibition of the antitumour immune response in the tumour microenvironment. Binding of PD-L1 to the PD-1 and B7.1 receptors found on T-cells and antigen presenting cells suppresses cytotoxic T-cell activity, T-cell proliferation and cytokine production.
Atezolizumab is an Fc-engineered, humanised immunoglobulin G1 (IgG1) monoclonal antibody that directly binds to PD-L1 and provides a dual blockade of the PD-1 and B7.1 receptors, releasing PD-L1/PD-1 mediated inhibition of the immune response, including reactivating the antitumour immune response without inducing antibody-dependent cellular cytotoxicity. Atezolizumab spares the PD-L2/PD-1 interaction allowing PD-L2/PD-1 mediated inhibitory signals to persist.
Clinical efficacy and safety
Urothelial carcinoma
IMvigor211 (GO29294): Randomised trial in locally advanced or metastatic UC patients previously treated with chemotherapy
A phase III, open-label, multi-centre, international, randomised study, (IMvigor211), was conducted to evaluate the efficacy and safety of atezolizumab compared with chemotherapy (investigator’s choice of vinflunine, docetaxel, or paclitaxel) in patients with locally advanced or metastatic UC who progressed during or following a platinum-containing regimen. This study excluded patients who had a history of autoimmune disease; active or corticosteroid-dependent brain metastases; administration of a live, attenuated vaccine within 28 days prior to enrolment; and administration of systemic immunostimulatory agents within 4 weeks or systemic immunosuppressive medicinal product within 2 weeks prior to enrolment. Tumour assessments were conducted every 9 weeks for the first 54 weeks, and every 12 weeks thereafter. Tumour specimens were evaluated prospectively for PD-L1 expression on tumour-infiltrating immune cells (IC) and the results were used to define the PD-L1 expression subgroups for the analyses described below.
A total of 931 patients were enrolled. Patients were randomised (1:1) to receive either atezolizumab or chemotherapy. Randomisation was stratified by chemotherapy (vinflunine vs. taxane), PD-L1 expression status on IC (<5% vs. ≥5%), number of prognostic risk factors (0 vs. 1-3), and liver metastases (yes vs. no). Prognostic risk factors included time from prior chemotherapy of <3 months, ECOG performance status >0 and haemoglobin <10 g/dL.
Atezolizumab was administered as a fixed dose of 1 200 mg by intravenous infusion every 3 weeks. No dose reduction of atezolizumab was allowed. Patients were treated until loss of clinical benefit as assessed by the investigator or unacceptable toxicity. Vinflunine was administered 320 mg/m² by intravenous infusion on day 1 of each 3-week cycle until disease progression or unacceptable toxicity. Paclitaxel was administered 175 mg/m² by intravenous infusion over 3 hours on day 1 of each 3-week cycle until disease progression or unacceptable toxicity. Docetaxel was administered 75 mg/m² by intravenous infusion on day 1 of each 3-week cycle until disease progression or unacceptable toxicity. For all treated patients, the median duration of treatment was 2.8 months for the atezolizumab arm, 2.1 months for the vinflunine and paclitaxel arms and 1.6 months for the docetaxel arm.
The demographic and baseline disease characteristics of the primary analysis population were well balanced between the treatment arms. The median age was 67 years (range: 31 to 88), and 77.1% of patients were male. The majority of patients were white (72.1%), 53.9% of patients within the chemotherapy arm received vinflunine, 71.4% of patients had at least one poor prognostic risk factor and 28.8% had liver metastases at baseline. Baseline ECOG performance status was 0 (45.6%) or 1 (54.4%). Bladder was the primary tumour site for 71.1% of patients and 25.4% of patients had upper tract UC. There were 24.2% of patients who received only prior platinum-containing adjuvant or neoadjuvant therapy and progressed within 12 months.
The primary efficacy endpoint for IMvigor211 is overall survival (OS). Secondary efficacy endpoints evaluated per investigator-assessed Response Evaluation Criteria in Solid Tumours (RECIST) v1.1 are objective response rate (ORR), progression-free survival (PFS), and duration of response (DOR). Comparisons with respect to OS between the treatment arm and control arm within the IC2/3, IC1/2/3, and ITT (Intention-to-treat, i.e. all comers) populations were tested using a hierarchical fixed-sequence procedure based on a stratified log-rank test at two-sided level of 5% as follows: step 1) IC2/3 population; step 2) IC1/2/3 population; step 3) all comers population. OS results for each of steps 2 and 3 could be formally tested for statistical significance only if the result in the preceding step was statistically significant.
The median survival follow-up is 17 months. The primary analysis of study IMvigor211 did not meet its primary endpoint of OS. Atezolizumab did not demonstrate a statistically significant survival benefit compared to chemotherapy in patients with previously treated, locally advanced or metastatic UC. Per the pre-specified hierarchical testing order, the IC2/3 population was tested first, with an OS HR of 0.87 (95% CI: 0.63, 1.21; median OS of 11.1 vs. 10.6 months for atezolizumab and chemotherapy respectively). The stratified log-rank p-value was 0.41 and therefore the results are considered not statistically significant in this population. As a consequence, no formal tests of statistical significance could be performed for OS in the IC1/2/3 or all comer populations, and results of those analyses would be considered exploratory. The key results in the all comer population are summarised in Table 4. The Kaplan-Meier curve for OS in the all comer population is presented in Figure 1.
An exploratory updated survival analysis was performed with a median duration of survival follow up of 34 months in the ITT population. The median OS was 8.6 months (95% CI: 7.8, 9.6) in the atezolizumab arm and 8.0 months (95% CI: 7.2, 8.6) in the chemotherapy arm with a hazard ratio of 0.82 (95% CI: 0.71, 0.94). Consistent with the trend observed at primary analysis for 12-month OS rates, numerically higher 24-month and 30-month OS rates were observed for patients in the atezolizumab arm compared with the chemotherapy arm in the ITT population. The percentage of patients alive at 24 months (KM estimate) was 12.7% in the chemotherapy arm and 22.5% in the atezolizumab arm; and at 30 months (KM estimate) was 9.8% in the chemotherapy arm and 18.1% in the atezolizumab arm.
Table 4. Summary of efficacy in all comers (IMvigor211):
| Efficacy endpoint | Atezolizumab (n=467) | Chemotherapy (n=464) |
|---|---|---|
| Primary efficacy endpoint | ||
| OS* | ||
| No. of deaths (%) | 324 (69.4%) | 350 (75.4%) |
| Median time to events (months) | 8.6 | 8.0 |
| 95% CI | 7.8, 9.6 | 7.2, 8.6 |
| Stratifiedǂ hazard ratio (95% CI) | 0.85 (0.73, 0.99) | |
| 12-month OS (%)** | 39.2% | 32.4% |
| Secondary and exploratory endpoints | ||
| Investigator-assessed PFS (RECIST v1.1) | ||
| No. of events (%) | 407 (87.2%) | 410 (88.4%) |
| Median duration of PFS (months) | 2.1 | 4.0 |
| 95% CI | 2.1, 2.2 | 3.4, 4.2 |
| Stratified hazard ratio (95% CI) | 1.10 (0.95, 1.26) | |
| Investigator-assessed ORR (RECIST v1.1) | n=462 | n=461 |
| No. of confirmed responders (%) | 62 (13.4%) | 62 (13.4%) |
| 95% CI | 10.45, 16.87 | 10.47, 16.91 |
| No. of complete response (%) | 16 (3.5%) | 16 (3.5%) |
| No. of partial response (%) | 46 (10.0%) | 46 (10.0%) |
| No. of stable disease (%) | 92 (19.9%) | 162 (35.1%) |
| Investigator-assessed DOR (RECIST v1.1) | n=62 | n=62 |
| Median in months*** | 21.7 | 7.4 |
| 95% CI | 13.0, 21.7 | 6.1, 10.3 |
CI = confidence interval; DOR = duration of response; ORR = objective response rate; OS = overall survival; PFS = progression-free survival; RECIST = Response Evaluation Criteria in Solid Tumours v1.1.
* An analysis of OS in the all comer population was performed based on the stratified log-rank test and the result is provided for descriptive purposes only (p=0.0378); according to the pre-specified analysis hierarchy, the p-value for the OS analysis in the all comer population cannot be considered statistically significant.
ǂ Stratified by chemotherapy (vinflunine vs. taxane), status on IC (<5% vs. ≥5%), number of prognostic risk factors (0 vs. 1-3), and liver metastases (yes vs. no).
** Based on Kaplan-Meier estimate
*** Responses were ongoing in 63% of responders in the atezolizumab arm and in 21% of responders in the chemotherapy arm.
Figure 1. Kaplan-Meier curve for overall survival (IMvigor211):
IMvigor210 (GO29293): Single-arm trial in previously untreated urothelial carcinoma patients who are ineligible for cisplatin therapy and in urothelial carcinoma patients previously treated with chemotherapy
A phase II, multi-centre, international, two-cohort, single-arm clinical trial, IMvigor210, was conducted in patients with locally advanced or metastatic UC (also known as urothelial bladder cancer).
The study enrolled a total of 438 patients and had two patient cohorts. Cohort 1 included previously untreated patients with locally advanced or metastatic UC who were ineligible or unfit for cisplatin-based chemotherapy or had disease progression at least 12 months after treatment with a platinum-containing neoadjuvant or adjuvant chemotherapy regimen. Cohort 2 included patients who received at least one platinum-based chemotherapy regimen for locally advanced or metastatic UC or had disease progression within 12 months of treatment with a platinum-containing neoadjuvant or adjuvant chemotherapy regimen.
In Cohort 1, 119 patients were treated with atezolizumab 1 200 mg by intravenous infusion every 3 weeks until disease progression. The median age was 73 years. Most patients were male (81%), and the majority of patients were White (91%).
Cohort 1 included 45 patients (38%) with ECOG performance status of 0, 50 patients (42%) with ECOG performance status of 1 and 24 patients (20%) with ECOG performance status of 2, 35 patients (29%) with no Bajorin risk factors (ECOG performance status ≥2 and visceral metastasis), 66 patients (56%) with one Bajorin risk factor and 18 patients (15 ) with two Bajorin risk factors, 84 patients (71) with impaired renal function (glomerular filtration rate [GFR] <60 mL/min), and 25 patients (21%) with liver metastasis.
The primary efficacy endpoint for Cohort 1 was confirmed objective response rate (ORR) as assessed by an independent review facility (IRF) using RECIST v1.1.
The primary analysis was performed when all patients had at least 24 weeks of follow-up. Median duration of treatment was 15.0 weeks and median duration of survival follow-up was 8.5 months in all comers. Clinically relevant IRF-assessed ORRs per RECIST v1.1 were shown; however, when compared to a pre-specified historical control response rate of 10%, statistical significance was not reached for the primary endpoint. The confirmed ORRs per IRF-RECIST v1.1 were 21.9% (95% CI: 9.3, 40.0) in patients with PD-L1 expression ≥5%, 18.8% (95% CI: 10.9, 29.0) in patients with PD-L1 expression ≥1%, and 19.3% (95% CI: 12.7, 27.6) in all comers. The median duration of response (DOR) was not reached in any PD-L1 expression subgroup or in all comers. OS was not mature with an event patient ratio of approximately 40%. Median OS for all patient subgroups (PD-L1 expression ≥5% and ≥1%) and in all comers was 10.6 months.
An updated analysis was performed with a median duration of survival follow-up of 17.2 months for Cohort 1 and is summarised in Table 5. The median DOR was not reached in any PD-L1 expression subgroup or in all comers.
Table 5. Summary of updated efficacy (IMvigor210 Cohort 1):
| Efficacy endpoint | PD-L1 expression of ≥5% in IC | PD-L1 expression of ≥1% in IC | All Comers |
|---|---|---|---|
| ORR (IRF-assessed; RECIST v1.1) | n=32 | n=80 | n=119 |
| No. of Responders () 95 CI | 9 (28.1%) 13.8, 46.8 | 19 (23.8%) 15.0, 34.6 | 27 (22.7%) 15.5, 31.3 |
| No. of complete response () 95 CI | 4 (12.5%) (3.5, 29.0) | 8 (10.0%) (4.4, 18.8) | 11 (9.2%) (4.7, 15.9) |
| No. of partial response () 95 CI | 5 (15.6%) (5.3, 32.8) | 11 (13.8%) (7.1, 23.3) | 16 (13.4%) (7.9, 20.9) |
| DOR (IRF-assessed; RECIST v1.1) | n=9 | n=19 | n=27 |
| Patients with event (%) | 3 (33.3%) | 5 (26.3%) | 8 (29.6%) |
| Median (months) (95% CI) | NE (11.1, NE) | NE (NE) | NE (14.1, NE) |
| PFS (IRF-assessed; RECIST v1.1) | n=32 | n=80 | n=119 |
| Patients with event (%) | 24 (75.0%) | 59 (73.8%) | 88 (73.9%) |
| Median (months) (95% CI) | 4.1 (2.3, 11.8) | 2.9 (2.1, 5.4) | 2.7 (2.1, 4.2) |
| OS | n=32 | n=80 | n=119 |
| Patients with event (%) | 18 (56.3%) | 42 (52.5%) | 59 (49.6%) |
| Median (months) (95% CI) | 12.3 (6.0, NE) | 14.1 (9.2, NE) | 15.9 (10.4, NE) |
| 1-year OS rate (%) | 52.4% | 54.8% | 57.2% |
CI = confidence interval; DOR=duration of response; IC = tumour-infiltrating immune cells; IRF = independent review facility; NE = not estimable; ORR = objective response rate; OS = overall survival; PFS = progression-free survival; RECIST = Response Evaluation Criteria in Solid Tumours v1.1.
At the time of the final analysis for Cohort 1, patients had a median survival follow-up time of 96.4 months. Median OS was 12.3 months (95% CI: 6.0, 49.8) in patients with PD-L1 expression ≥5% (patients who are included in the therapeutic indication).
In Cohort 2, the co-primary efficacy endpoints were confirmed ORR as assessed by an IRF using RECIST v1.1 and investigator-assessed ORR according to Modified RECIST (mRECIST) criteria. There were 310 patients treated with atezolizumab 1 200 mg by intravenous infusion every 3 weeks until loss of clinical benefit. The primary analysis of Cohort 2 was performed when all patients had at least 24 weeks of follow-up. The study met its co-primary endpoints in Cohort 2, demonstrating statistically significant ORRs per IRF-assessed RECIST v1.1 and investigator-assessed mRECIST compared to a pre-specified historical control response rate of 10%.
An analysis was also performed with a median duration of survival follow-up of 21.1 months for Cohort 2. The confirmed ORRs per IRF-RECIST v1.1 were 28.0% (95% CI: 19.5, 37.9) in patients with PD-L1 expression ≥5%, 19.3% (95% CI: 14.2, 25.4) in patients with PD-L1 expression ≥1%, and 15.8% (95% CI: 11.9, 20.4) in all comers. The confirmed ORR per investigator-assessed mRECIST was 29.0% (95% CI: 20.4, 38.9) in patients with PD-L1 expression ≥5%, 23.7% (95% CI: 18.1, 30.1) in patients with PD-L1 expression ≥1%, and 19.7% (95% CI: 15.4, 24.6) in all comers. The rate of complete response per IRF-RECIST v1.1 in the all comer population was 6.1% (95% CI: 3.7, 9.4). For Cohort 2, median DOR was not reached in any PD-L1 expression subgroup or in all comers, however was reached in patients with PD-L1 expression <1% (13.3 months; 95% CI 4.2, NE). The OS rate at 12 months was 37% in all comers.
At the time of the final analysis for Cohort 2, patients had a median survival follow-up time of 46.2 months. Median OS was 11.9 months (95% CI: 9.0, 22.8) in patients with PD-L1 expression ≥5%, 9.0 months (95% CI: 7.1, 11.1) in patients with PD-L1 expression ≥1%, and 7.9 months (95% CI: 6.7, 9.3) in all comers.
IMvigor130 (WO30070): Phase III study of atezolizumab monotherapy and in combination with platinum-based chemotherapy in patients with untreated locally advanced or metastatic urothelial carcinoma
A phase III, multi-centre, randomised, placebo-controlled, partially blinded (Arms A and C only) study, IMvigor130, was conducted to evaluate the efficacy and safety of atezolizumab + platinumbased combination chemotherapy (i.e., either cisplatin or carboplatin with gemcitabine), Arm A, or atezolizumab monotherapy (Arm B, open-label arm) versus placebo + platinum-based combination chemotherapy (Arm C) in patients with locally advanced or metastatic UC who had not received prior systemic therapy in the metastatic setting. The co-primary efficacy outcomes were investigator-assessed progression-free survival (PFS) in Arm A versus Arm C and overall survival (OS) in Arm A versus C and then Arm B versus C, analyzed in a hierarchical fashion. Overall survival was not statistically significant for the comparison of Arm A versus Arm C, and thus no further formal testing could be conducted per the pre-defined hierarchical testing order.
Based on an independent Data Monitoring Committee (iDMC) recommendation following an early review of survival data, accrual of patients on the atezolizumab monotherapy treatment arm whose tumours had a low PD-L1 expression (less than 5% of immune cells staining positive for PD-L1 by immunohistochemistry using VENTANA PD-L1 [SP142] assay) was stopped after observing decreased overall survival for this subgroup at an unplanned early analysis, however, this occurred after the vast majority of patients had already been enrolled.
Out of 719 patients enrolled in the atezolizumab monotherapy (n=360) and chemotherapy alone (n=359) arms, 50 and 43 patients, respectively, were cisplatin-ineligible by Galsky criteria and had tumours with high PD-L1 expression (≥5% of immune cells staining positive for PD-L1 by immunohistochemistry using VENTANA PD-L1 [SP142] assay). In an exploratory analysis in this subgroup of patients, the unstratified HR for OS was 0.56 (95% CI: 0.34, 0.91). The median OS was 18.6 months (95% CI: 14.0, 49.4) in the atezolizumab monotherapy arm vs. 10.0 months (95% CI: 7.4, 18.1) in the chemotherapy alone arm (see Figure 2).
Figure 2. Kaplan-Meier Plot of Overall Survival in Cisplatin-ineligible patients whose tumours are PD-L1 high (Arm B vs. Arm C):
Non-small cell lung cancer
Adjuvant treatment of early-stage NSCLC
IMpower010 (GO29527): Randomised phase III trial in patients with resected NSCLC after cisplatin-based chemotherapy
A phase III, open label, multi-centre, randomised study, GO29527 (IMpower010), was conducted to evaluate the efficacy and safety of atezolizumab for the adjuvant treatment of patients with stage IB (tumours ≥4 cm) – IIIA NSCLC (per the Union for International Cancer Control/American Joint Committee on Cancer staging system, 7th edition).
The following selection criteria define patients with high risk of recurrence who are included in the therapeutic indication and are reflective of the patient population with stage II – IIIA according to the 7th edition staging system:
Tumour size ≥5 cm; or tumours of any size that are either accompanied by N1 or N2 status; or tumours that are invasive of thoracic structures (directly invade the parietal pleura, chest wall, diaphragm, phrenic nerve, mediastinal pleura, parietal pericardium, mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, oesophagus, vertebral body, carina); or tumours that involve the main bronchus <2 cm distal to the carina but without involvement of the carina; or tumours that are associated with atelectasis or obstructive pneumonitis of the entire lung; or tumours with separate nodule(s) in the same lobe or different ipsilateral lobe as the primary.
The study did not include patients who had N2 status with tumours invading the mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, oesophagus, vertebral body, carina, or with separate tumour nodule(s) in a different ipsilateral lobe.
A total of 1 280 enrolled patients had complete tumour resection and were eligible to receive up to 4 cycles of cisplatin-based chemotherapy. The cisplatin-based chemotherapy regimens are described in Table 6.
Table 6. Adjuvant chemotherapy regimens (IMpower010):
| Adjuvant cisplatin-based chemotherapy: Cisplatin 75 mg/m² intravenous on Day 1 of each 21 day cycle with one of the following treatment regimens | Vinorelbine 30 mg/m² intravenous, Days 1 and 8 |
| Docetaxel 75 mg/m² intravenous, Day 1 | |
| Gemcitabine 1 250 mg/m² intravenous, Days 1 and 8 | |
| Pemetrexed 500 mg/m² intravenous, Day 1(non-squamous) |
After completion of cisplatin-based chemotherapy (up to four cycles), a total of 1 005 patients were randomised in a 1:1 ratio to receive atezolizumab (Arm A) or best supportive care (BSC) (Arm B). Atezolizumab was administered as a fixed dose of 1 200 mg by IV infusion every 3 weeks for 16 cycles unless there was disease recurrence or unacceptable toxicity. Randomisation was stratified by sex, stage of disease, histology, and PD-L1 expression.
Patients were excluded if they had a history of autoimmune disease; administration of a live, attenuated vaccine within 28 days prior to randomisation; administration of systemic immunostimulatory agents within 4 weeks or systemic immunosuppressive medications within 2 weeks prior to randomisation. Tumour assessments were conducted at baseline of the randomisation phase and every 4 months for the first year following Cycle 1, Day 1 and then every 6 months until year five, then annually thereafter.
The demographics and baseline disease characteristics in the ITT population were well balanced between the treatment arms. The median age was 62 years (range: 26 to 84), and 67% of patients were male. The majority of patients were White (73%), and 24% were Asian. Most patients were current or previous smokers (78%) and baseline ECOG performance status in patients was 0 (55%) or 1 (44%). Overall, 12% of patients had stage IB, 47% had stage II and 41% had stage IIIA disease. The percentage of patients who had tumours with PD-L1 expression ≥1% and ≥50% on TC as measured by the VENTANA PD-L1 (SP263) Assay was 55% and 26%, respectively.
The primary efficacy outcome measure was disease-free survival (DFS) as assessed by the investigator. DFS was defined as the time from the date of randomisation to the date of occurrence of any of the following: first documented recurrence of disease, new primary NSCLC, or death due to any cause, whichever occurred first. The primary efficacy objective was to evaluate DFS in the PD-L1 ≥1% TC stage II – IIIA patient population. Key secondary efficacy objectives were to evaluate DFS in the PD-L1 ≥50% TC stage II – IIIA patient population and overall survival (OS) in the ITT population.
At the time of the interim DFS analysis, the study met its primary endpoint. In the analysis of patients with PD-L1 ≥50% TC stage II – IIIA without EGFR mutations or ALK rearrangements (n=209), an improvement in DFS in the atezolizumab arm was observed compared to the BSC arm. Results were consistent at the time of the final DFS analysis, with median follow up time of 65 months.
The key efficacy results for DFS and OS in the PD-L1 ≥50% TC stage II – IIIA patient population, without EGFR mutations and ALK rearrangements, are summarised in Table 7. The Kaplan-Meier curve for DFS is presented in Figure 3.
Table 7. Summary of efficacy in the PD-L1 expression ≥50% TC stage II – IIIA patient population without EGFR mutations or ALK rearrangements (IMpower010):
| Efficacy endpoint | Arm A (Atezolizumab) | Arm B (Best supportive care) |
|---|---|---|
| Investigator-assessed DFS* | n=106 | n=103 |
| No. of events (%) | 34 (32.1%) | 55 (53.4%) |
| Median duration of DFS (months) | NE | 42.9 |
| 95% CI | (NE) | (32.0, NE) |
| Stratifiedǂ hazard ratio (95% CI) | 0.52 (0.33, 0.80) | |
| OS* | n=106 | n=103 |
| No. of events (%) | 22 (20.8%) | 41 (39.8%) |
| Median OS (months) | NE | 87.1 |
| 95% CI | (NE) | (72.0, NE) |
| Stratifiedǂ hazard ratio (95% CI) | 0.47 (0.28, 0.80) | |
DFS = Disease-free survival; CI = confidence interval; NE = not estimable
* Updated DFS and OS analysis at clinical cut-off 26 January 2024
ǂ Stratified by stage, sex, and histology.
Figure 3. Kaplan-Meier curve for disease-free survival in the PD-L1 expression ≥50% TC stage II – IIIA patient population without EGFR mutations or ALK rearrangements (IMpower010):
The observed DFS improvement in the atezolizumab arm compared with the BSC arm was consistently shown across the majority of pre-specified subgroups in the PD-L1 ≥50% TC stage II – IIIA patient population without EGFR mutations or ALK rearrangements, including both non-squamous NSCLC patients (unstratified HR of 0.40, 95% CI: 0.23, 0.70; median DFS NE vs. 36.8 months) and squamous NSCLC patients (unstratified HR of 0.67, 95% CI: 0.34, 1.32; median DFS could not be estimated).
First-line treatment of advanced NSCLC
IMpower150 (GO29436): Randomised phase III trial in chemotherapy-naïve patients with metastatic non-squamous NSCLC, in combination with paclitaxel and carboplatin with or without bevacizumab
A phase III, open-label, multi-centre, international, randomised study, IMpower150, was conducted to evaluate the efficacy and safety of atezolizumab in combination with paclitaxel and carboplatin, with or without bevacizumab, in chemotherapy-naïve patients with metastatic non-squamous NSCLC.
Patients were excluded if they had history of autoimmune disease, administration of a live, attenuated vaccine within 28 days prior to randomisation, administration of systemic immunostimulatory agents within 4 weeks or systemic immunosuppressive medicinal product within 2 weeks prior to randomisation, active or untreated CNS metastases, clear tumour infiltration into the thoracic great vessels or clear cavitation of pulmonary lesions, as seen on imaging. Tumour assessments were conducted every 6 weeks for the first 48 weeks following Cycle 1, Day 1 and then every 9 weeks thereafter. Tumour specimens were evaluated for PD-L1 expression on tumour cells (TC) and tumour-infiltrating immune cells (IC) and the results were used to define the PD-L1 expression subgroups for the analyses described below.
A total of 1 202 patients were enrolled and were randomised (1:1:1) to receive one of the treatment regimens described in Table 8. Randomisation was stratified by sex, presence of liver metastases and PD-L1 tumour expression on TC and IC.
Table 8. Intravenous treatment regimens (IMpower150):
| Treatment regimen | Induction (Four or Six 21-day cycles) | Maintenance (21-day cycles) |
|---|---|---|
| A | Atezolizumaba (1 200 mg) + paclitaxel (200 mg/m²)b,c + carboplatinc (AUC 6) | Atezolizumaba (1 200 mg) |
| B | Atezolizumaba (1 200 mg) + bevacizumabd (15 mg/kg bw) + paclitaxel (200 mg/m²)b,c + carboplatinc (AUC 6) | Atezolizumaba (1 200 mg) + bevacizumabd (15 mg/kg bw) |
| C | Bevacizumabd (15 mg/kg bw) + paclitaxel (200 mg/m²)b,c + carboplatinc (AUC 6) | Bevacizumabd (15 mg/kg bw) |
a Atezolizumab is administered until loss of clinical benefit as assessed by the investigator.
b The paclitaxel starting dose for patients of Asian race/ethnicity was 175 mg/m² due to higher overall level of haematologic toxicities in patients from Asian countries compared with those from non-Asian countries.
c Paclitaxel and carboplatin are administered until completion of 4 or 6 cycles, or progressive disease, or unacceptable toxicity whichever occurs first.
d Bevacizumab is administered until progressive disease or unacceptable toxicity.
The demographics and baseline disease characteristics of the study population were well balanced between the treatment arms. The median age was 63 years (range: 31 to 90), and 60% of patients were male. The majority of patients were white (82%). Approximately 10% of patients had known EGFR mutation, 4% had known ALK rearrangements, 14% had liver metastasis at baseline, and most patients were current or previous smokers (80%). Baseline ECOG performance status was 0 (43%) or 1 (57%). 51% of patients' tumours had PD-L1 expression of ≥1% TC or ≥1% IC and 49% of patients' tumours had PD-L1 expression of <1% TC and <1% IC.
At the time of the final analysis for PFS, patients had a median follow up time of 15.3 months. The ITT population, including patients with EGFR mutations or ALK rearrangements who should have been previously treated with tyrosine kinase inhibitors, demonstrated clinically meaningful PFS improvement in Arm B as compared to Arm C (HR of 0.61, 95% CI: 0.52, 0.72; median PFS 8.3 vs. 6.8 months).
At the time of the interim OS analysis, patients had a median follow-up of 19.7 months. The key results from this analysis as well as from the updated PFS analysis in the ITT population are summarised in Tables 9 and 10. The Kaplan-Meier curve for OS in the ITT population is presented in Figure 4. Figure 5 summarises the results of OS in the ITT and PD-L1 subgroups. Updated PFS results are also presented in Figures 6 and 7.
Table 9. Summary of updated efficacy in the ITT population (IMpower150):
| Efficacy endpoint | Arm A (Atezolizumab + Paclitaxel + Carboplatin) | Arm B (Atezolizumab + Bevacizumab + Paclitaxel + Carboplatin) | Arm C (Bevacizumab + Paclitaxel + Carboplatin) |
|---|---|---|---|
| Secondary Endpoints# | |||
| Investigator-assessed PFS (RECIST v1.1)* | n=402 | n=400 | n=400 |
| No. of events (%) | 330 (82.1%) | 291 (72.8%) | 355 (88.8%) |
| Median duration of PFS (months) | 6.7 | 8.4 | 6.8 |
| 95% CI | (5.7, 6.9) | (8.0, 9.9) | (6.0, 7.0) |
| Stratified hazard ratio‡^ (95% CI) p-value1,2 | 0.91 (0.78, 1.06) 0.2194 | 0.59 (0.50, 0.69) <0.0001 | --- |
| 12-month PFS (%) | 24 | 38 | 20 |
| OS interim analysis* | n=402 | n=400 | n=400 |
| No. of deaths (%) | 206 (51.2%) | 192 (48.0%) | 230 (57.5%) |
| Median time to events (months) | 19.5 | 19.8 | 14.9 |
| 95% CI | (16.3, 21.3) | (17.4, 24.2) | (13.4, 17.1) |
| Stratified hazard ratio‡^ (95% CI) p-value1,2 | 0.85 (0.71, 1.03) 0.0983 | 0.76 (0.63, 0.93) 0.006 | --- |
| 6-month OS (%) | 84 | 85 | 81 |
| 12-month OS (%) | 66 | 68 | 61 |
| Investigator-assessed Overall Best Response3* (RECIST 1.1) | n=401 | n=397 | n=393 |
| No. of responders (%) | 163 (40.6%) | 224 (56.4%) | 158 (40.2%) |
| 95% CI | (35.8, 45.6) | (51.4, 61.4) | (35.3, 45.2) |
| No. of complete response (%) | 8 (2.0%) | 11 (2.8%) | 3 (0.8%) |
| No. of partial response (%) | 155 (38.7%) | 213 (53.7%) | 155 (39.4%) |
| Investigator-assessed DOR* (RECIST v1.1) | n=163 | n=224 | n=158 |
| Median in months | 8.3 | 11.5 | 6.0 |
| 95% CI | (7.1, 11.8) | (8.9, 15.7) | (5.5, 6.9) |
# Primary efficacy endpoints were PFS and OS and they were analysed in the ITT-wild-type (WT) population, i.e. excluding patients with EGFR mutations or ALK rearrangements.
1 Based on the stratified log-rank test
2 For informational purposes; in the ITT population, comparisons between Arm B and Arm C as well as between Arm A and Arm C were not formally tested yet as per the pre-specified analysis hierarchy
3 Overall best response for complete response and partial response
‡ Stratified by sex, presence of liver metastases and PD-L1 tumour expression on TC and IC
^ The Arm C is the comparison group for all hazard ratios
* Updated PFS analysis and interim OS analysis at clinical cut-off 22 January 2018
PFS = progression-free survival; RECIST = Response Evaluation Criteria in Solid Tumours v1.1.
CI = confidence interval; DOR = duration of response; OS = overall survival.
Table 10. Summary of updated efficacy for Arm A vs. Arm B in the ITT population (IMpower150):
| Efficacy endpoint | Arm A (Atezolizumab + Paclitaxel + Carboplatin) | Arm B (Atezolizumab + Bevacizumab + Paclitaxel + Carboplatin) |
|---|---|---|
| Investigator-assessed PFS (RECIST v1.1)* | n=402 | n=400 |
| No. of events (%) | 330 (82.1%) | 291 (72.8%) |
| Median duration of PFS (months) | 6.7 | 8.4 |
| 95% CI | (5.7, 6.9) | (8.0, 9.9) |
| Stratified hazard ratio‡^ (95% CI) p-value1,2 | 0.67 (0.57, 0.79) <0.0001 | |
| OS interim analysis* | n=402 | n=400 |
| No. of deaths (%) | 206 (51.2%) | 192 (48.0%) |
| Median time to events (months) | 19.5 | 19.8 |
| 95% CI | (16.3, 21.3) | (17.4, 24.2) |
| Stratified hazard ratio‡^ (95% CI) p-value1,2 | 0.90 (0.74, 1.10) 0.3000 | |
1 Based on the stratified log-rank test.
2 For informational purposes; in the ITT population, comparisons between Arm A and Arm B were not included in the pre-specified analysis hierarchy.
‡ Stratified by sex, presence of liver metastases and PD-L1 expression on TC and IC
* Updated PFS analysis and interim OS analysis at clinical cut-off 22 January 2018
^ The Arm A is the comparison group for all hazard ratios
Figure 4. Kaplan-Meier curve for overall survival in the ITT population (IMpower150):
Figure 5. Forest plot of overall survival by PD-L1 expression in the ITT population, Arm B vs. C (IMpower150):
Figure 6. Kaplan-Meier curve for PFS in the ITT population (IMpower150):
Figure 7. Forest plot of progression free survival by PD-L1 expression in the ITT population, Arm B vs. C (IMpower150):
In Arm B as compared to Arm C, pre-specified subgroup analyses from the interim OS analysis showed an OS improvement for patients with EGFR mutations or ALK rearrangements (hazard ratio [HR] of 0.54, 95% CI: 0.29, 1.03; median OS not reached vs. 17.5 months), and liver metastases (HR of 0.52, 95% CI: 0.33, 0.82; median OS 13.3 vs. 9.4 months). PFS improvements were also shown in patients with EGFR mutations or ALK rearrangements (HR of 0.55, 95% CI: 0.35, 0.87; median PFS 10.0 vs. 6.1 months) and liver metastases (HR of 0.41, 95% CI: 0.26, 0.62; median PFS 8.2 vs. 5.4 months). OS results were similar for patients aged <65 and ≥65 subgroups, respectively. Data for patients ≥75 years of age are too limited to draw conclusions on this population. For all subgroup analyses, formal statistical testing was not planned.
IMpower130 (GO29537): Randomised phase III trial in chemotherapy-naïve patients with metastatic non-squamous NSCLC, in combination with nab-paclitaxel and carboplatin
A phase III, open-label, randomised study, GO29537 (IMpower130), was conducted to evaluate the efficacy and safety of atezolizumab in combination with nab-paclitaxel and carboplatin, in chemotherapy-naïve patients with metastatic non-squamous NSCLC. Patients with EGFR mutations or ALK rearrangements should have been previously treated with tyrosine kinase inhibitors.
Patients were staged according to the American Joint Committee on Cancer (AJCC) 7th edition. Patients were excluded if they had a history of autoimmune disease, administration of live, attenuated vaccine within 28 days prior to randomisation, administration of immunostimulatory agents within 4 weeks or systemic immunosuppressive medicinal products within 2 weeks prior to randomisation, and active or untreated CNS metastases. Patients who had prior treatment with CD137 agonists or immune checkpoint blockade therapies (anti-PD-1, and anti-PD-L1 therapeutic antibodies) were not eligible. However, patients who had prior anti-CTLA-4 treatment could be enrolled, as long as the last dose was received at least 6 weeks prior to randomisation, and there was no history of severe immune-mediated adverse events from anti-CTLA-4 (NCI CTCAE Grades 3 and 4). Tumour assessments were conducted every 6 weeks for the first 48 weeks following Cycle 1, then every 9 weeks thereafter. Tumour specimens were evaluated for PD-L1 expression on tumour cells (TC) and tumour infiltrating immune cells (IC) and the results were used to define the PD-L1 expression subgroups for the analyses described below.
Patients, including those with EGFR mutations or ALK rearrangements, were enrolled and were randomised in a 2:1 ratio to receive one of the treatment regimens described in Table 11. Randomisation was stratified by sex, presence of liver metastases and PD-L1 expression on TC and IC. Patients receiving treatment regimen B were able to crossover and receive atezolizumab monotherapy following disease progression.
Table 11. Intravenous treatment regimens (IMpower130):
| Treatment Regimen | Induction (Four or six 21-day cycles) | Maintenance (21-day cycles) |
|---|---|---|
| A | Atezolizumab (1 200 mg)a + nab-paclitaxel (100 mg/m²)b,c + carboplatin (AUC 6)c | Atezolizumab (1 200 mg)a |
| B | Nab-paclitaxel (100 mg/m²)b,c + carboplatin (AUC 6)c | Best supportive care or pemetrexed |
a Atezolizumab is administered until loss of clinical benefit as assessed by investigator.
b Nab-paclitaxel is administered on days 1, 8, and 15 of each cycle.
c Nab-paclitaxel and carboplatin are administered until completion of 4-6 cycles, or progressive disease or unacceptable toxicity whichever occurs first.
The demographics and baseline disease characteristics of the study population defined as ITT-WT (n=679) were well balanced between the treatment arms. The median age was 64 years (range: 18 to 86 years). The majority of the patients were male (59%) and white (90%). Fourteen point seven percent of patients had liver metastases at baseline, and most patients were current or previous smokers (90%). The majority of patients had a baseline ECOG performance status of 1 (59%) and PD-L1 expression <1% (approximately 52%). Among 107 Arm B patients who had a response status of stable disease, partial response, or complete response after induction therapy, 40 received pemetrexed switch maintenance therapy.
The primary analysis was conducted in all patients, excluding those with EGFR mutations or ALK rearrangements, defined as ITT-WT population (n=679). Patients had a median survival follow up time of 18.6 months and showed improved OS and PFS with atezolizumab, nab-paclitaxel and carboplatin as compared to the control. The key results are summarised in Table 12 and Kaplan-Meier curves for OS and PFS are presented in Figures 8 and 10, respectively. The exploratory results of OS and PFS by PD-L1 expression are summarised in Figures 9 and 11, respectively. Patients with liver metastases did not show improved PFS or OS with atezolizumab, nab-paclitaxel and carboplatin, compared to nab-paclitaxel and carboplatin (HR of 0.93, 95% CI: 0.59, 1.47 for PFS and HR of 1.04, 95% CI: 0.63, 1.72 for OS, respectively).
Fifty-nine percent of patients in the nab-paclitaxel and carboplatin arm received any cancer immunotherapy after disease progression, which includes atezolizumab as crossover treatment (41% of all patients), compared to 7.3% of patients in the atezolizumab, nab paclitaxel and carboplatin arm.
In an exploratory analysis with longer follow up (median: 24.1 months), the median OS for both arms was unchanged relative to the primary analysis, with HR = 0.82 (95% CI: 0.67, 1.01).
Table 12. Summary of efficacy from IMpower130 in the primary analysis (ITT-WT population):
| Efficacy endpoints | Arm A Atezolizumab + nab-paclitaxel + carboplatin | Arm B Nab-paclitaxel + carboplatin |
|---|---|---|
| Co-primary endpoints | ||
| OS | n=451 | n=228 |
| No. of deaths (%) | 226 (50.1%) | 131 (57.5%) |
| Median time to events (months) | 18.6 | 13.9 |
| 95% CI | (16.0, 21.2) | (12.0, 18.7) |
| Stratified hazard ratio‡ (95% CI) | 0.79 (0.64, 0.98) | |
| p-value | 0.033 | |
| 12-month OS (%) | 63 | 56 |
| Investigator-assessed PFS (RECIST v1.1) | n=451 | n=228 |
| No. of events (%) | 347 (76.9%) | 198 (86.8%) |
| Median duration of PFS (months) | 7.0 | 5.5 |
| 95% CI | (6.2, 7.3) | (4.4, 5.9) |
| Stratified hazard ratio‡ (95% CI) | 0.64 (0.54, 0.77) | |
| p-value | <0.0001 | |
| 12-month PFS (%) | 29% | 14% |
| Other endpoints | ||
| Investigator-assessed ORR (RECIST v1.1)^ | n=447 | n=226 |
| No. of confirmed responders (%) | 220 (49.2%) | 72 (31.9%) |
| 95% CI | (44.5, 54.0) | (25.8, 38.4) |
| No. of complete response (%) | 11 (2.5%) | 3 (1.3%) |
| No. of partial response (%) | 209 (46.8%) | 69 (30.5%) |
| Investigator-assessed confirmed DOR (RECIST 1.1)^ | n=220 | n=72 |
| Median in months | 8.4 | 6.1 |
| 95% CI | (6.9, 11.8) | (5.5, 7.9) |
‡ Stratified by sex and PD-L1 expression on TC and IC
^ Confirmed ORR and DoR are exploratory endpoints
PFS = progression-free survival; RECIST=Response Evaluation Criteria in Solid Tumours v1.1.; CI = confidence interval; ORR = objective response rate; DOR = duration of response; OS = overall survival
Figure 8. Kaplan-Meier curves for overall survival (IMpower130):
Figure 9. Forest plot of overall survival by PD-L1 expression (IMpower130):
Figure 10. Kaplan-Meier curves for progression free survival (IMpower130):
Figure 11. Forest plot of progression free survival by PD-L1 expression (IMpower130):
IMpower110 (GO29431): Randomised phase III trial in chemotherapy-naïve patients with metastatic NSCLC
A phase III, open-label, multi-centre, randomised study, IMpower110, was conducted to evaluate the efficacy and safety of atezolizumab in chemotherapy-naïve patients with metastatic NSCLC. Patients had PD-L1 expression ≥1% TC (PD-L1 stained ≥1% of tumour cells) or ≥1% IC (PD-L1 stained tumour-infiltrating immune cells covering ≥1% of the tumour area) based on the VENTANA PD-L1 (SP142) Assay.
A total of 572 patients were randomised in a 1:1 ratio to receive atezolizumab (Arm A) or chemotherapy (Arm B). Atezolizumab was administered as a fixed dose of 1 200 mg by intravenous infusion every 3 weeks until loss of clinical benefit as assessed by the investigator or unacceptable toxicity. The chemotherapy regimens are described in Table 13. Randomisation was stratified by sex, ECOG performance status, histology, and PD-L1 tumour expression on TC and IC.
Table 13. Chemotherapy intravenous treatment regimens (IMpower110):
| Treatment regimen | Induction (Four or Six 21-day cycles) | Maintenance (21-day cycles) |
|---|---|---|
| B (Non- squamous) | Cisplatina (75 mg/m²) + pemetrexeda (500 mg/m²) OR carboplatina (AUC 6) + pemetrexeda (500 mg/m²) | Pemetrexedb,d (500 mg/m²) |
| B (Squamous) | Cisplatina (75 mg/m²) + gemcitabinea,c (1 250 mg/m²) OR carboplatina (AUC 5) + gemcitabinea,c (1 000 mg/m²) | Best supportive cared |
a Cisplatin, carboplatin, pemetrexed and gemcitabine are administered until completion of 4 or 6 cycles, or progressive disease, or unacceptable toxicity.
b Pemetrexed is administered as maintenance regimen every 21 days until progressive disease or unacceptable toxicity.
c Gemcitabine is administered on days 1 and 8 of each cycle.
d No crossover was allowed from the control arm (platinum-based chemotherapy) to the atezolizumab arm (Arm A).
Patients were excluded if they had a history of autoimmune disease; administration of a live, attenuated vaccine within 28 days prior to randomisation, administration of systemic immunostimulatory agents within 4 weeks or systemic immunosuppressive medicinal products within 2 weeks prior to randomisation, active or untreated CNS metastases. Tumour assessments were conducted every 6 weeks for the first 48 weeks following Cycle 1, Day 1 and then every 9 weeks thereafter.
The demographics and baseline disease characteristics in patients with PD-L1 expression ≥1% TC or ≥1% IC who do not have EGFR mutations or ALK rearrangements (n=554) were well balanced between the treatment arms. The median age was 64.5 years (range: 30 to 87), and 70% of patients were male. The majority of patients were white (84%) and Asian (14%). Most patients were current or previous smokers (87%) and baseline ECOG performance status in patients was 0 (36%) or 1 (64%). Overall, 69% of patients had non-squamous disease and 31% of patients had squamous disease. The demographics and baseline disease characteristics in patients with high PD-L1 expression (PD-L1 ≥50% TC or ≥10% IC) who do not have with EGFR mutations or ALK rearrangements (n=205) were generally representative of the broader study population and were balanced between the treatment arms.
The primary endpoint was overall survival (OS). At the time of the interim OS analysis, patients with high PD-L1 expression excluding those with EGFR mutations or ALK rearrangements (n=205) showed statistically significant improvement in OS for the patients randomised to atezolizumab (Arm A) as compared with chemotherapy (Arm B) (HR of 0.59, 95% CI: 0.40, 0.89; median OS of 20.2 months vs 13.1 months) with a two-sided p-value of 0.0106. The median survival follow-up time in patients with high PD-L1 expression was 15.7 months.
In an exploratory OS analysis with longer follow up (median: 31.3 months) for these patients, the median OS for the atezolizumab arm was unchanged relative to the primary OS interim analysis (20.2 months) and was 14.7 months for the chemotherapy arm (HR of 0.76, 95% CI: 0.54, 1.09). The key results at the exploratory analysis are summarised in Table 14. The Kaplan-Meier curves for OS and PFS in patients with high PD-L1 expression are presented in Figures 12 and 13. A higher proportion of patients experienced death within the first 2.5 months in the atezolizumab arm (16/107, 15.0%) as compared to the chemotherapy arm (10/98, 10.2%). No specific factor(s) associated with early deaths could be identified.
Table 14. Summary of efficacy in patients with high PD-L1 expression ≥50% TC or ≥10% IC (IMpower110):
| Efficacy endpoints | Arm A (Atezolizumab) | Arm B (Chemotherapy) |
|---|---|---|
| Primary endpoint | ||
| Overall survival | n=107 | n=98 |
| No. of deaths (%) | 64 (59.8%) | 64 (65.3%) |
| Median time to events (months) | 20.2 | 14.7 |
| 95% CI | (17.2, 27.9) | (7.4, 17.7) |
| Stratified hazard ratio‡ (95% CI) | 0.76 (0.54, 1.09) | |
| 12-month OS (%) | 66.1 | 52.3 |
| Secondary endpoints | ||
| Investigator-assessed PFS (RECIST v1.1) | n=107 | n=98 |
| No. of events (%) | 82 (76.6%) | 87 (88.8%) |
| Median duration of PFS (months) | 8.2 | 5.0 |
| 95% CI | (6.8, 11.4) | (4.2, 5.7) |
| Stratified hazard ratio‡ (95% CI) | 0.59 (0.43, 0.81) | |
| 12-month PFS (%) | 39.2 | 19.2 |
| Investigator-assessed ORR (RECIST 1.1) | n=107 | n=98 |
| No. of responders (%) | 43 (40.2%) | 28 (28.6%) |
| 95% CI | (30.8, 50.1) | (19.9, 38.6) |
| No. of complete response (%) | 1 (0.9%) | 2 (2.0%) |
| No. of partial response (%) | 42 (39.3%) | 26 (26.5%) |
| Investigator-assessed DOR (RECIST 1.1) | n=43 | n=28 |
| Median in months | 38.9 | 8.3 |
| 95% CI | (16.1, NE) | (5.6, 11.0) |
‡ Stratified by sex and ECOG performance status (0 vs. 1)
PFS = progression-free survival; RECIST = Response Evaluation Criteria in Solid Tumours v1.1; CI = confidence interval; ORR = objective response rate; DOR = duration of response; OS = overall survival; NE = not estimable.
Figure 12. Kaplan-Meier curve for overall survival in patients with high PD-L1 expression ≥50% TC or ≥10% IC (IMpower110):
Figure 13. Kaplan-Meier curve for progression free survival in patients with high PD-L1 expression ≥50% TC or ≥10% IC (IMpower110):
The observed OS improvement in the atezolizumab arm compared with the chemotherapy arm was consistently shown across subgroups in patients with high PD-L1 expression including both non-squamous NSCLC patients (hazard ratio [HR] of 0.62, 95% CI: 0.40, 0.96; median OS 20.2 vs. 10.5 months) and squamous NSCLC patients (HR of 0.56, 95% CI: 0.23, 1.37; median OS not reached vs. 15.3 months). Data for patients ≥75 years of age and patients who were never smokers are too limited to draw conclusions in these subgroups.
IPSOS study (MO29872): Randomised phase III trial in patients with treatment-naïve locally advanced unresectable or metastatic NSCLC who are ineligible for platinum-based chemotherapy
A phase III, open label, randomised, controlled study, MO29872 (IPSOS), was conducted to evaluate the efficacy and safety of atezolizumab compared with a single-agent chemotherapy regimen (vinorelbine or gemcitabine by investigator choice) in treatment-naïve patients with advanced or recurrent (Stage IIIB [according to the AJCC 7 th edition] not amenable to multimodality treatment) or metastatic (Stage IV) NSCLC who were considered ineligible for platinum-based chemotherapy.
The following selection criteria define patients ineligible for platinum-based chemotherapy who are included in the therapeutic indication: Patients >80 years of age, or with an ECOG performance status (PS) of 3, or patients with an ECOG PS 2 in combination with relevant comorbidities, or of older age (≥70 years) in combination with relevant comorbidities. Relevant comorbidities are related to cardiac disorders, nervous system disorders, psychiatric disorders, vascular disorders, renal disorders, metabolism and nutrition disorders, or pulmonary disorders contraindicating treatment with platinum-based therapy, as assessed by the treating physician.
The study excluded patients younger than 70 years who had an ECOG PS of 0 or 1; patients with active or untreated CNS metastases; administration of live, attenuated vaccine within 4 weeks prior to randomisation; administration of systemic immunostimulatory or systemic immunosuppressive medicinal products within 4 weeks prior to randomisation. Patients with EGFR mutations or ALK rearrangements were also excluded from the study. Patients were eligible regardless of their tumour PD-L1 status.
Patients were randomised in a 2:1 ratio to receive atezolizumab (Arm A) or chemotherapy (Arm B). Atezolizumab was administered as a fixed dose of 1 200 mg by intravenous infusion every 3 weeks. The chemotherapy regimens are described in Table 15. Treatment was administered until disease progression per RECIST v1.1 or unacceptable toxicity. Randomisation was stratified by histology (squamous/non-squamous), PD-L1 expression (PD-L1 IHC status as measured by the VENTANA PD-L1 (SP142) assay: TC3 or IC3 vs TC0/1/2 and IC0/1/2 vs unknown) and brain metastases (yes/no).
Table 15. Treatment regimens (IPSOS):
| Treatment Regimen | |
|---|---|
| A | Atezolizumab 1 200 mg by IV infusion on Day 1 of every 21-day cycle. |
| B | Vinorelbine: IV infusion at 25-30 mg/m² or oral administration at 60-80 mg/m² on Days 1 and 8 of each 21-day cycle or on Days 1, 8 and 15 of each 28-day cycle or weekly administration or Gemcitabine: IV infusion at 1 000-1 250 mg/m² on Days 1 and 8 of each 21-day cycle or on Days 1, 8 and 15 of each 28-day cycle. |
A total of 453 patients were enrolled in the study (ITT population). The population predominantly comprised White (65.8%) and male (72.4%) patients. The median age of patients was 75 years and 72.8% of patients were aged 70 years or older. The proportion of patients with ECOG PS of 0, 1, 2 and 3 was 1.5%, 15.0%, 75.9%, and 7.5%, respectively. Overall, 13.7% of patients had stage IIIB disease not amenable to multimodality treatment and 86.3% had stage IV disease. The percentage of patients who had tumours with PD-L1 expression TC< 1%, 1-49% and ≥ 50% as measured by the VENTANA PD-L1 (SP263) assay was 46.8%, 28.7% and 16.6%, respectively, while 7.9% of patients had an unknown PD-L1 expression status.
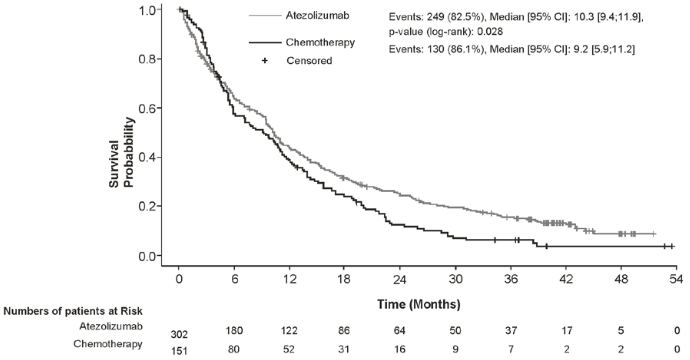
The primary endpoint of the study was overall survival (OS). At the time of the final OS analysis, the median follow-up was 41.0 months. Efficacy results are presented in Table 16 and Figure 14.
Table 16. Summary of efficacy for NSCLC patients ineligible for platinum-based chemotherapy (IPSOS):
| Efficacy endpoint | Atezolizumab (n=302) | Chemotherapy (n=151) |
|---|---|---|
| Primary endpoint | ||
| OS | ||
| No. of events (%) | 249 (82.5%) | 130 (86.1%) |
| Median time to events (months) (95% CI) | 10.3 (9.4, 11.9) | 9.2 (5.9, 11.2) |
| Stratified hazard ratio (95% CI)ǂ | 0.78 (0.63, 0.97) | |
| p-value (Stratified Log-rank) | p=0.028 | |
| Secondary endpoints | ||
| Investigator-assessed PFS (RECIST 1.1) | ||
| No. of events (%) | 276 (91.4%) | 138 (91.4%) |
| Median duration of PFS (months) (95% CI) | 4.2 (3.7, 5.5) | 4.0 (2.9, 5.4) |
| Stratified hazard ratio (95% CI)ǂ | 0.87 (0.70, 1.07) | |
| ORR (RECIST 1.1) | ||
| No. of confirmed responders (%) | 51 (16.9%) | 12 (7.9%) |
| DOR (RECIST 1.1) | ||
| Median in months (95% CI) | 14.0 (8.1, 20.3) | 7.8 (4.8, 9.7) |
CI = confidence interval; DOR = duration of response; ORR = objective response rate; OS = overall survival; PFS = progression-free survival; RECIST = Response Evaluation Criteria in Solid Tumours v1.1.
ǂ Estimated hazard ratio and 95% CI obtained from Cox model with treatment group as covariate. For the stratified analysis, histologic subtype, PD-L1 IHC status and brain metastases (yes/no) were added as stratification factors.
Figure 14. Kaplan-Meier curves for overall survival for NSCLC patients ineligible for platinum-based chemotherapy (IPSOS):
Second-line treatment of NSCLC
OAK (GO28915): Randomised phase III trial in locally advanced or metastatic NSCLC patients previously treated with chemotherapy
A phase III, open-label, multi-centre, international, randomised study, OAK, was conducted to evaluate the efficacy and safety of atezolizumab compared with docetaxel in patients with locally advanced or metastatic NSCLC who progressed during or following a platinum-containing regimen. This study excluded patients who had a history of autoimmune disease, active or corticosteroid-dependent brain metastases, administration of a live, attenuated vaccine within 28 days prior to enrolment, administration of systemic immunostimulatory agents within 4 weeks or systemic immunosuppressive medicinal product within 2 weeks prior to enrolment. Tumour assessments were conducted every 6 weeks for the first 36 weeks, and every 9 weeks thereafter. Tumour specimens were evaluated prospectively for PD-L1 expression on tumour cells (TC) and tumour-infiltrating immune cells (IC).
A total of 1 225 patients were enrolled and per the analysis plan the first 850 randomised patients were included in the primary efficacy analysis. Randomisation was stratified by PD-L1 expression status on IC, by the number of prior chemotherapy regimens, and by histology. Patients were randomised (1:1) to receive either atezolizumab or docetaxel.
Atezolizumab was administered as a fixed dose of 1 200 mg by intravenous infusion every 3 weeks. No dose reduction was allowed. Patients were treated until loss of clinical benefit as assessed by the investigator. Docetaxel was administered 75 mg/m² by intravenous infusion on day 1 of each 3-week cycle until disease progression. For all treated patients, the median duration of treatment was 2.1 months for the docetaxel arm and 3.4 months for the atezolizumab arm.
The demographic and baseline disease characteristics of the primary analysis population were well balanced between the treatment arms. The median age was 64 years (range: 33 to 85), and 61% of patients were male. The majority of patients were white (70%). Approximately three-quarters of patients had non-squamous histology (74%), 10% had known EGFR mutation, 0.2% had known ALK rearrangements, 10% had CNS metastases at baseline, and most patients were current or previous smokers (82%). Baseline ECOG performance status was 0 (37%) or 1 (63%). Seventy-five percent of patients received only one prior platinum-based therapeutic regimen.
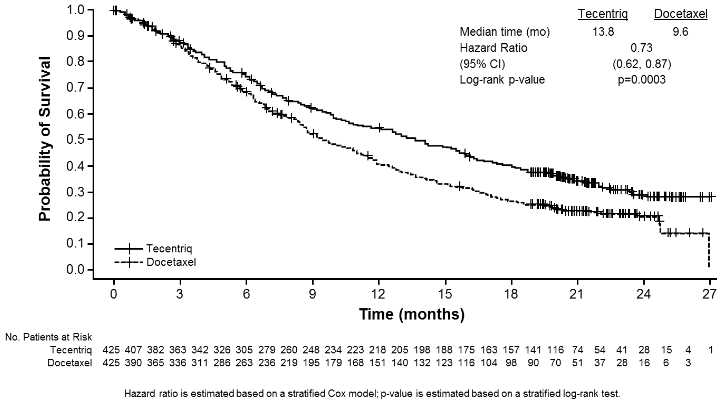
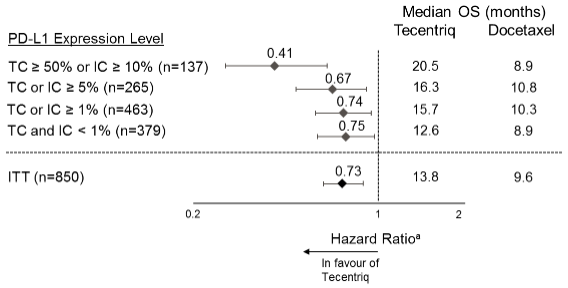
The primary efficacy endpoint was OS. The key results of this study with a median survival follow-up of 21 months are summarised in Table 17. Kaplan-Meier curves for OS in the ITT population are presented in Figure 15. Figure 16 summarises the results of OS in the ITT and PD-L1 subgroups, demonstrating OS benefit with atezolizumab in all subgroups, including those with PD-L1 expression <1% in TC and IC.
Table 17. Summary of efficacy in the primary analysis population (all comers)* (OAK):
| Efficacy endpoint | Atezolizumab (n=425) | Docetaxel (n=425) |
|---|---|---|
| Primary efficacy endpoint | ||
| OS | ||
| No. of deaths (%) | 271 (64%) | 298 (70%) |
| Median time to events (months) | 13.8 | 9.6 |
| 95% CI | (11.8, 15.7) | (8.6, 11.2) |
| Stratifiedǂ hazard ratio (95% CI) | 0.73 (0.62, 0.87) | |
| p-value** | 0.0003 | |
| 12-month OS (%)*** | 218 (55%) | 151 (41%) |
| 18-month OS (%)*** | 157 (40%) | 98 (27%) |
| Secondary endpoints | ||
| Investigator-assessed PFS (RECIST v1.1) | ||
| No. of events (%) | 380 (89%) | 375 (88%) |
| Median duration of PFS (months) | 2.8 | 4.0 |
| 95% CI | (2.6, 3.0) | (3.3, 4.2) |
| Stratified hazard ratio (95% CI) | 0.95 (0.82, 1.10) | |
| Investigator-assessed ORR (RECIST v1.1) | ||
| No. of responders (%) | 58 (14%) | 57 (13%) |
| 95% CI | (10.5, 17.3) | (10.3, 17.0) |
| Investigator-assessed DOR (RECIST v1.1) | n=58 | n=57 |
| Median in months | 16.3 | 6.2 |
| 95% CI | (10.0, NE) | (4.9, 7.6) |
CI = confidence interval; DOR = duration of response; NE = not estimable; ORR = objective response rate; OS = overall survival; PFS = progression-free survival; RECIST = Response Evaluation Criteria in Solid Tumours v1.1.
* The primary analysis population consists of the first 850 randomised patients.
ǂ Stratified by PD-L1 expression in tumour infiltrating immune cells, the number of prior chemotherapy regimens, and histology.
** Based on the stratified log-rank test.
*** Based on Kaplan-Meier estimates.
Figure 15. Kaplan-Meier curve for overall survival in the primary analysis population (all comers) (OAK):
Figure 16. Forest plot of overall survival by PD-L1 expression in the primary analysis population (OAK):
a Stratified HR for ITT and TC or IC ≥1%. Unstratified HR for other exploratory subgroups.
An improvement in OS was observed with atezolizumab compared to docetaxel in both non-squamous NSCLC patients (hazard ratio [HR] of 0.73, 95% CI: 0.60, 0.89; median OS of 15.6 vs. 11.2 months for atezolizumab and docetaxel, respectively) and squamous NSCLC patients (HR of 0.73, 95% CI: 0.54, 0.98; median OS of 8.9 vs. 7.7 months for atezolizumab and docetaxel, respectively). The observed OS improvement was consistently demonstrated across subgroups of patients including those with brain metastases at baseline (HR of 0.54, 95% CI: 0.31, 0.94; median OS of 20.1 vs. 11.9 months for atezolizumab and docetaxel respectively) and patients who were never smokers (HR of 0.71, 95% CI: 0.47, 1.08; median OS of 16.3 vs. 12.6 months for atezolizumab and docetaxel, respectively). However, patients with EGFR mutations did not show improved OS with atezolizumab compared to docetaxel (HR of 1.24, 95% CI: 0.71, 2.18; median OS of 10.5 vs. 16.2 months for atezolizumab and docetaxel, respectively).
Prolonged time to deterioration of patient-reported pain in chest as measured by the EORTC QLQ-LC13 was observed with atezolizumab compared to docetaxel (HR of 0.71, 95% CI: 0.49, 1.05; median not reached in either arm). The time to deterioration in other lung cancer symptoms (i.e. cough, dyspnoea, and arm/shoulder pain) as measured by the EORTC QLQ-LC13 was similar between atezolizumab and docetaxel. These results should be interpreted with caution due to the open-label design of the study.
POPLAR (GO28753): Randomised phase II trial in locally advanced or metastatic NSCLC patients previously treated with chemotherapy
A phase II, multi-centre, international, randomised, open-label, controlled study, POPLAR, was conducted in patients with locally advanced or metastatic NSCLC who progressed during or following a platinum-containing regimen, regardless of PD-L1 expression. The primary efficacy outcome was overall survival. A total of 287 patients were randomised 1:1 to receive either atezolizumab (1 200 mg by intravenous infusion every 3 weeks until loss of clinical benefit) or docetaxel (75 mg/m² by intravenous infusion on day 1 of each 3-week cycle until disease progression). Randomisation was stratified by PD-L1 expression status on IC, by the number of prior chemotherapy regimens and by histology. An updated analysis with a total of 200 deaths observed and a median survival follow-up of 22 months showed a median OS of 12.6 months in patients treated with atezolizumab, vs. 9.7 months in patients treated with docetaxel (HR of 0.69, 95% CI: 0.52, 0.92). ORR was 15.3% vs. 14.7% and median DOR was 18.6 months vs. 7.2 months for atezolizumab vs. docetaxel, respectively.
Small cell lung cancer
IMpower133 (GO30081): Randomised phase I/III trial in patients with chemotherapy-naïve extensive-stage SCLC, in combination with carboplatin and etoposide
A Phase I/III, randomised, multicentre, double-blind, placebo-controlled study, IMpower133, was conducted to evaluate the efficacy and safety of atezolizumab in combination with carboplatin and etoposide in patients with chemotherapy-naïve ES-SCLC.
Patients were excluded if they had active or untreated CNS metastases; history of autoimmune disease; administration of live, attenuated vaccine within 4 weeks prior to randomisation; administration of systemic immunosuppressive medicinal products within 1 week prior to randomisation. Tumour assessments were conducted every 6 weeks for the first 48 weeks following Cycle 1, Day 1 and then every 9 weeks thereafter. Patients who met established criteria and who agreed to be treated beyond disease progression had tumour assessments conducted every 6 weeks until treatment discontinuation.
A total of 403 patients were enrolled and randomised (1:1) to receive one of the treatment regimens described in Table 18. Randomisation was stratified by sex, ECOG performance status, and presence of brain metastases.
Table 18. Intravenous treatment regimens (IMpower133):
| Treatment regimen | Induction (Four 21-Day Cycles) | Maintenance (21-Day Cycles) |
|---|---|---|
| A | atezolizumab (1 200 mg)a + carboplatin (AUC 5)b + etoposide (100 mg/m²)b,c | atezolizumab (1 200 mg)a |
| B | placebo + carboplatin (AUC 5)b + etoposide (100 mg/m²)b,c | placebo |
a Atezolizumab was administered until loss of clinical benefit as assessed by investigator.
b Carboplatin and etoposide were administered until completion of 4 cycles, or progressive disease or unacceptable toxicity, whichever occurs first.
c Etoposide was administered on day 1, 2 and 3 of each cycle.
The demographic and baseline disease characteristics of the study population were well balanced between the treatment arms. The median age was 64 years (range: 26 to 90 years) with 10% of patients ≥75 years of age. The majority of patients were male (65%), white (80%), and 9% had brain metastases and most patients were current or previous smokers (97%). Baseline ECOG performance status was 0 (35%) or 1 (65%).
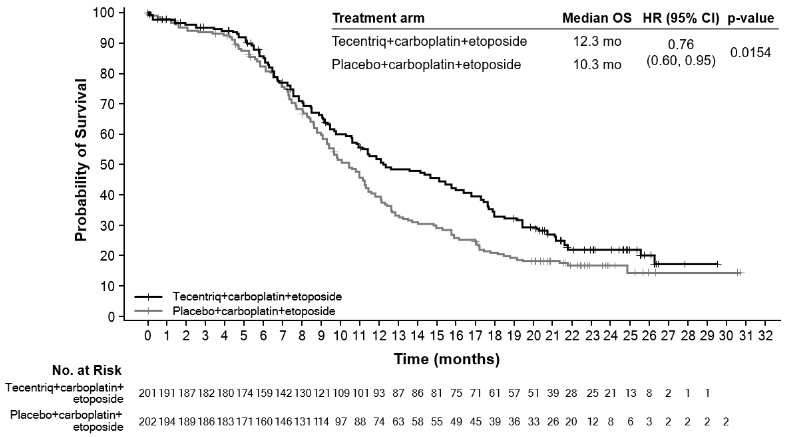
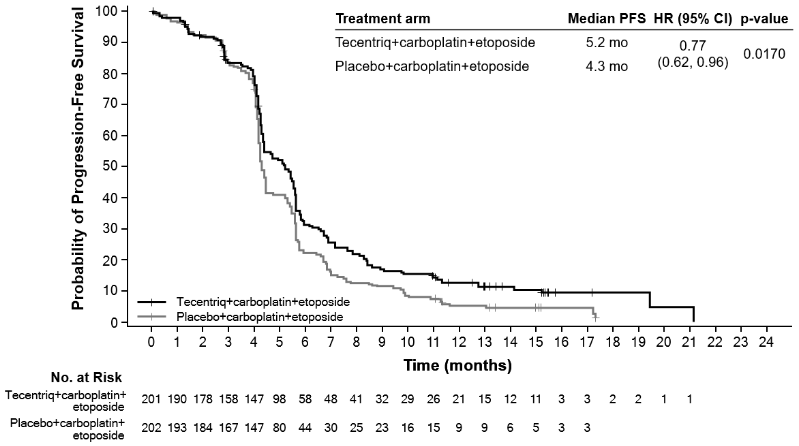
At the time of the primary analysis, patients had a median survival follow up time of 13.9 months. A statistically significant improvement in OS was observed with atezolizumab in combination with carboplatin and etoposide compared to the control arm (HR of 0.70, 95% CI: 0.54, 0.91; median OS of 12.3 months vs. 10.3 months). In the exploratory OS final analysis with longer follow up (median: 22.9 months), the median OS for both arms was unchanged relative to the primary OS interim analysis. The PFS, ORR and DOR results from the primary analysis as well as the exploratory OS final analysis results are summarised in Table 19. Kaplan-Meier curves for OS and PFS are presented in Figures 17 and 18. Data for patients with brain metastases are too limited to draw conclusions on this population.
Table 19. Summary of efficacy (IMpower133):
| Key efficacy endpoints | Arm A (Atezolizumab + carboplatin + etoposide) | Arm B (Placebo + carboplatin + etoposide) |
|---|---|---|
| Co-primary endpoints | ||
| OS analysis* | n=201 | n=202 |
| No. of deaths (%) | 142 (70.6%) | 160 (79.2%) |
| Median time to events (months) | 12.3 | 10.3 |
| 95% CI | (10.8, 15.8) | (9.3, 11.3) |
| Stratified hazard ratio‡ (95% CI) | 0.76 (0.60, 0.95) | |
| p-value | 0.0154*** | |
| 12-month OS (%) | 51.9 | 39.0 |
| Investigator-assessed PFS (RECIST v1.1)** | n=201 | n=202 |
| No. of events (%) | 171 (85.1%) | 189 (93.6%) |
| Median duration of PFS (months) | 5.2 | 4.3 |
| 95% CI | (4.4, 5.6) | (4.2, 4.5) |
| Stratified hazard ratio‡ (95% CI) | 0.77 (0.62, 0.96) | |
| p-value | 0.0170 | |
| 6-month PFS (%) | 30.9 | 22.4 |
| 12-month PFS (%) | 12.6 | 5.4 |
| Other endpoints | ||
| Investigator-assessed ORR (RECIST 1.1)**^ | n=201 | n=202 |
| No. of responders (%) | 121 (60.2%) | 130 (64.4%) |
| 95% CI | (53.1, 67.0) | (57.3, 71.0) |
| No. of complete response (%) | 5 (2.5%) | 2 (1.0%) |
| No. of partial response (%) | 116 (57.7%) | 128 (63.4%) |
| Investigator-assessed DOR (RECIST 1.1)**^ | n=121 | n=130 |
| Median in months | 4.2 | 3.9 |
| 95% CI | (4.1, 4.5) | (3.1, 4.2) |
PFS = progression-free survival; RECIST = Response Evaluation Criteria in Solid Tumours v1.1.; CI = confidence interval; ORR = objective response rate; DOR = duration of response; OS = overall survival
‡ Stratified by sex and ECOG performance status
* Exploratory OS final analysis at clinical cut-off 24 January 2019
** PFS, ORR and DOR analyses at clinical cut-off 24 April 2018
*** For descriptive purposes only
^ Confirmed ORR and DoR are exploratory endpoints
Figure 17. Kaplan-Meier curve for overall survival (IMpower133):
Figure 18. Kaplan-Meier curve for progression-free survival (IMpower133):
Triple-negative breast cancer
IMpassion130 (WO29522): Randomised phase III trial in locally advanced or metastatic TNBC patients previously untreated for metastatic disease
A phase III, double-blind, two-arm, multi-centre, international, randomised, placebo-controlled study, IMpassion130, was conducted to evaluate the efficacy and safety of atezolizumab in combination with nab-paclitaxel, in patients with unresectable locally advanced or metastatic TNBC who had not received prior chemotherapy for metastatic disease. Patients had to be eligible for taxane monotherapy (i.e. absence of rapid clinical progression, life-threatening visceral metastases, or need for rapid symptom and/or disease control) and were excluded if they had received prior chemotherapy in the neoadjuvant or adjuvant setting within the last 12 months, a history of autoimmune disease; administration of a live, attenuated vaccine within 4 weeks prior to randomisation, administration of systemic immunostimulatory agents within 4 weeks or systemic immunosuppressive medicinal products within 2 weeks prior to randomisation; untreated, symptomatic or corticosteroid-dependent brain metastases. Tumour assessments were performed every 8 weeks (± 1 week) for the first 12 months after Cycle 1, day 1 and every 12 weeks (± 1 week) thereafter.
A total of 902 patients were enrolled and stratified by presence of liver metastases, prior taxane treatment, and by PD-L1 expression status in tumour-infiltrating immune cells (IC) (PD-L1 stained tumour-infiltrating immune cells [IC] <1% of tumour area vs. ≥1% of the tumour area) assessed by the VENTANA PD-L1 (SP142) Assay.
Patients were randomised to receive atezolizumab 840 mg or placebo by intravenous infusions on days 1 and 15 of every 28-day cycle, plus nab-paclitaxel (100 mg/m²) administered via intravenous infusion on days 1, 8 and 15 of every 28-day cycle. Patients received treatment until radiographic disease progression per RECIST v1.1, or unacceptable toxicity. Treatment with atezolizumab could be continued when nab-paclitaxel was stopped due to unacceptable toxicity. The median number of treatment cycles was 7 for atezolizumab and 6 for nab-paclitaxel in each treatment arm.
The demographic and baseline disease characteristics of the study population were well balanced between the treatment arms. Most patients were women (99.6%), 67.5% were white and 17.8% Asian. The median age was 55 years (range: 20-86). Baseline ECOG performance status was 0 (58.4%) or 1 (41.3%). Overall, 41% of enrolled patients had PD-L1 expression ≥1%, 27% had liver metastases and 7% asymptomatic brain metastases at baseline. Approximately half the patients had received a taxane (51%) or anthracycline (54%) in the (neo)adjuvant setting. Patient demographics and baseline tumour disease in patients with PD-L1 expression ≥1% were generally representative of the broader study population.
The co-primary efficacy endpoints included investigator-assessed progression free survival (PFS) in the ITT population and in patients with PD-L1 expression ≥1% per RECIST v1.1 as well as overall survival (OS) in the ITT population and in patients with PD-L1 expression ≥1%. Secondary efficacy endpoints included objective response rate (ORR) and duration of response (DOR) per RECIST v1.1.
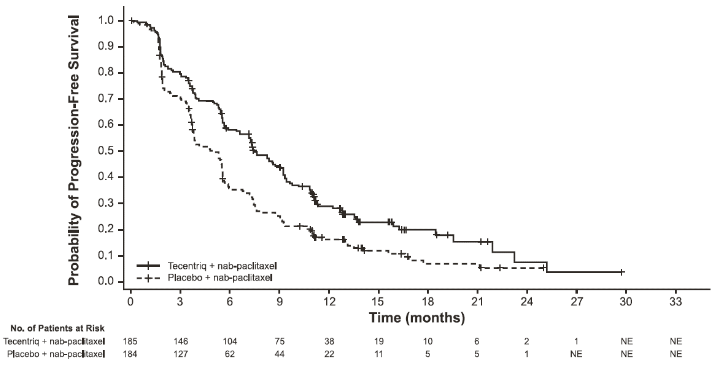
PFS, ORR and DOR results of IMpassion130 for patients with PD-L1 expression ≥1% at the time of the final analysis for PFS with a median survival follow up of 13 months are summarised in Table 20 with Kaplan-Meier curves for PFS in Figure 19. Patients with PD-L1 expression <1% did not show improved PFS when atezolizumab was added to nab-paclitaxel (HR of 0.94, 95% CI 0.78, 1.13).
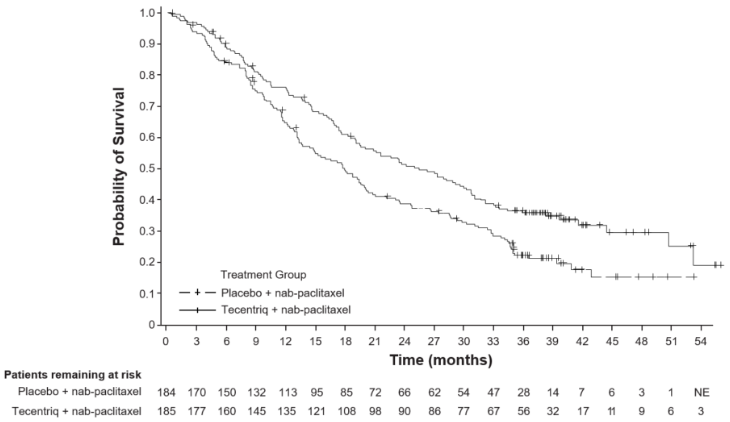
The final OS analysis was performed in patients with PD-L1 expression ≥1% with a median follow up of 19.12 months. OS results are presented in Table 20 and Kaplan-Meier curves in Figure 20. Patients with PD-L1 expression <1% did not show improved OS when atezolizumab was added to nab-paclitaxel (HR of 1.02, 95% CI 0.84, 1.24).
Exploratory subgroup analyses were performed in patients with PD-L1 expression ≥1%, exploring prior (neo)adjuvant treatment, BRCA1/2 mutation and asymptomatic brain metastases at baseline.
In patients who had received prior (neo) adjuvant treatment (n=242), the hazard ratio for primary (final) PFS was 0.79 and 0.77 for final OS while in patients who had not received prior (neo)adjuvant treatment (n=127), the hazard ratio for primary (final) PFS was 0.44 and 0.54 for final OS.
In the IMpassion130 study, of the 614 patients tested, 89 (15%) carried pathogenic BRCA1/2 mutations. From the PD-L1+/BRCA1/2 mutant subgroup, 19 patients received atezolizumab plus nabpaclitaxel and 26 placebo plus nab-paclitaxel. Based on exploratory analysis and acknowledging the small sample size, the presence of BRCA1/2 mutation does not seem to impact the PFS clinical benefit of atezolizumab and nab-paclitaxel.
There was no evidence of efficacy in patients with asymptomatic brain metastases at baseline, although the number of patients treated was small; the median PFS was 2.2 months in the atezolizumab plus nab-paclitaxel arm (n=15) compared to 5.6 months in the placebo plus nabpaclitaxel arm (n=11) (HR 1.40; 95% CI 0.57, 3.44).
Table 20. Summary of efficacy in patients with PD-L1 expression ≥1% (IMpassion130):
| Key efficacy endpoints | Atezolizumab + nab- paclitaxel | Placebo + nab-paclitaxel |
|---|---|---|
| Primary efficacy endpoints | n=185 | n=184 |
| Investigator-assessed PFS (RECIST v1.1) – Primary analysis3 | ||
| No. of events (%) | 138 (74.6%) | 157 (85.3%) |
| Median duration of PFS (months) | 7.5 | 5.0 |
| 95% CI | (6.7, 9.2) | (3.8, 5.6) |
| Stratified hazard ratio‡ (95% CI) | 0.62 (0.49, 0.78) | |
| p-value1 | <0.0001 | |
| 12-month PFS (%) | 29.1 | 16.4 |
| Investigator-assessed PFS (RECIST v1.1) – Updated exploratory analysis4 | ||
| No. of events (%) | 149 (80.5%) | 163 (88.6%) |
| Median duration of PFS (months) | 7.5 | 5.3 |
| 95% CI | (6.7, 9.2) | (3.8, 5.6) |
| Stratified hazard ratio‡ (95% CI) | 0.63 (0.50-0.80) | |
| p-value1 | <0.0001 | |
| 12-month PFS (%) | 30.3 | 17.3 |
| OS1,2,5 | ||
| No. of deaths (%) | 120 (64.9%) | 139 (75.5%) |
| Median time to events (months) | 25.4 | 17.9 |
| 95% CI | (19.6, 30.7) | (13.6, 20.3) |
| Stratified hazard ratio‡ (95% CI) | 0.67 (0.53, 0.86) | |
| Secondary and exploratory endpoints | ||
| Investigator-assessed ORR (RECIST 1.1)3 | n=185 | n=183 |
| No. of responders (%) | 109 (58.9%) | 78 (42.6%) |
| 95% CI | (51.5, 66.1) | (35.4, 50.1) |
| No. of complete response (%) | 19 (10.3%) | 2 (1.1%) |
| No. of partial response (%) | 90 (48.6%) | 76 (41.5%) |
| No. of stable disease | 38 (20.5%) | 49 (26.8%) |
| Investigator-assessed DOR3 | n=109 | n=78 |
| Median in months | 8.5 | 5.5 |
| 95% CI | (7.3, 9.7) | (3.7, 7.1) |
1 Based on the stratified log-rank test.
2 OS comparisons between treatment arms in patients with PD-L1 expression ≥1% were not formally tested, as per the pre-specified analysis hierarchy.
3 Per final analysis for PFS, ORR, DOR and first interim analysis for OS at clinical cut off 17th April 2018
4 Per exploratory PFS analysis at clinical cut off January 2nd 2019
5 Per final analysis for OS at clinical cut off April 14th 2020
‡ Stratified by presence of liver metastases, and by prior taxane treatment.
PFS = progression-free survival; RECIST = Response Evaluation Criteria in Solid Tumours v1.1.; CI = confidence interval; ORR = objective response rate; DOR = duration of response; OS=overall survival, NE = not estimable
Figure 19. Kaplan-Meier curve for progression free survival in patients with PD-L1 expression ≥1% (IMpassion130):
Figure 20. Kaplan-Meier curve for overall survival in patients with PD-L1 expression ≥1% (IMpassion130):
The time to deterioration (a sustained ≥10-point decline from baseline score) of patient-reported global health status/health-related quality of life as measured by the EORTC QLQ-C30 was similar in each treatment group indicating that all patients maintained their baseline HRQoL for a comparable duration of time.
Hepatocellular carcinoma
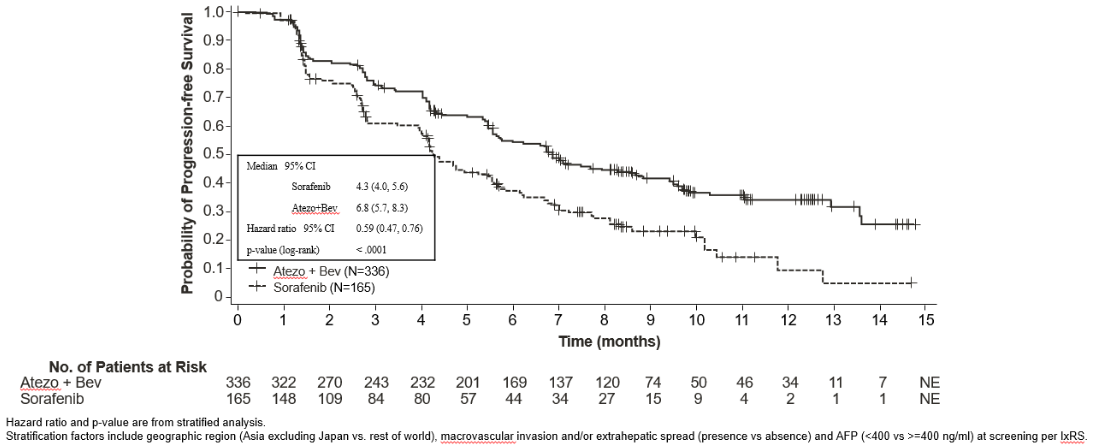
IMbrave150 (YO40245): Randomised phase III trial in patients with unresectable HCC who have not received prior systemic therapy, in combination with bevacizumab
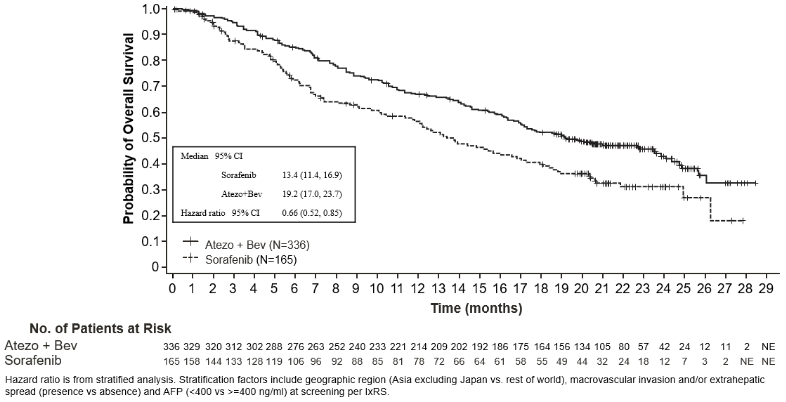
A phase III, randomised, multi-centre, international, open-label study, IMbrave150, was conducted to evaluate the efficacy and safety of atezolizumab in combination with bevacizumab, in patients with locally advanced or metastatic and/or unresectable HCC, who have not received prior systemic treatment. A total of 501 patients were randomised (2:1) to receive either atezolizumab (1 200 mg) and 15 mg/kg bw of bevacizumab every 3 weeks administered by intravenous infusion, or sorafenib 400 mg orally twice per day. Randomisation was stratified by geographic region, macrovascular invasion and/or extrahepatic spread, baseline α-fetoprotein (AFP) and ECOG performance status. Patients in both arms received treatment until loss of clinical benefit, or unacceptable toxicity. Patients could discontinue either atezolizumab or bevacizumab (e.g., due to adverse events) and continue on single-agent therapy until loss of clinical benefit or unacceptable toxicity associated with the single-agent.
The study enrolled adults whose disease was not amenable to or progressed after surgical and/or locoregional therapies, were Child-Pugh A, ECOG 0/1, and who had not received prior systemic treatment. Bleeding (including fatal events) is a known adverse reaction with bevacizumab and upper gastrointestinal bleeding is a common and life threatening complication in patients with HCC. Hence, patients were required to be evaluated for the presence of varices within 6 months prior to treatment, and were excluded if they had variceal bleeding within 6 months prior to treatment, untreated or incompletely treated varices with bleeding or high risk of bleeding. For patients with active hepatitis B, HBV DNA <500 IU/mL was required within 28 days prior to initiation of study treatment, and standard anti-HBV treatment for a minimum of 14 days prior to study entry and for the length of study.
Patients were also excluded if they had moderate or severe ascites; history of hepatic encephalopathy; known fibrolamellar HCC; sarcomatoid HCC, mixed cholangiocarcinoma and HCC; active co-infection of HBV and HCV; history of autoimmune disease; administration of a live, attenuated vaccine within 4 weeks prior to randomization; administration of systemic immunostimulatory agents within 4 weeks or systemic immunosuppressive medicinal products within 2 weeks prior to randomization; untreated or corticosteroid-dependent brain metastases. Tumour assessments were performed every 6 weeks for the first 54 weeks following Cycle 1, Day 1, then every 9 weeks thereafter.
The demographic and baseline disease characteristics of the study population were well balanced between the treatment arms. The median age was 65 years (range: 26 to 88 years) and 83% were male. The majority of patients were Asian (57%) and white (35%). 40% were from Asia (excluding Japan), while 60% were from rest of world. Approximately 75% of patients presented with macrovascular invasion and/or extrahepatic spread and 37% had a baseline AFP ≥400 ng/mL. Baseline ECOG performance status was 0 (62%) or 1 (38%). The primary risk factors for the development of HCC were Hepatitis B virus infection in 48% of patients, Hepatitis C virus infection in 22% of patients, and non-viral disease in 31% of patients. HCC was categorized as Barcelona Clinic Liver Cancer (BCLC) stage C in 82% of patients, stage B in 16% of patients, and stage A in 3% of patients.
The co-primary efficacy endpoints were OS and IRF-assessed PFS according to RECIST v1.1. At the time of the primary analysis, patients had a median survival follow up time of 8.6 months. The data demonstrated a statistically significant improvement in OS and PFS as assessed by IRF per RECIST v1.1 with atezolizumab + bevacizumab compared to sorafenib. A statistically significant improvement was also observed in confirmed objective response rate (ORR) by IRF per RECIST v1.1 and HCC modified RECIST (mRECIST). The key efficacy results from the primary analysis are summarized in Table 19.
A descriptive updated efficacy analysis was performed with a median survival follow up time of 15.6 months. The median OS was 19.2 months (95% CI: 17.0, 23.7) in the atezolizumab + bevacizumab arm versus 13.4 months (95% CI: 11.4, 16.9) in the sorafenib arm with a HR of 0.66 (95% CI: 0.52, 0.85). The median PFS by IRF-assessment per RECIST v1.1 was 6.9 months (95% CI: 5.8, 8.6) in the atezolizumab + bevacizumab arm versus 4.3 months (95% CI: 4.0, 5.6) in the sorafenib arm with a HR of 0.65 (95% CI: 0.53, 0.81).
The IRF-assessed ORR per RECIST v1.1 was 29.8% (95% CI: 24.8, 35.0) in the atezolizumab + bevacizumab arm and 11.3% (95% CI: 6.9, 17.3) in the sorafenib arm. The median duration of response (DOR) by IRF-assessment per RECIST v1.1 in confirmed responders was 18.1 months (95% CI: 14.6, NE) in the atezolizumab + bevacizumab arm compared to 14.9 months (95% CI: 4.9, 17.0) in the sorafenib arm.
Kaplan-Meier curves for OS (updated analysis) and PFS (primary analysis) are presented in Figures 21 and 22, respectively.
Table 21. Summary of efficacy (IMbrave150 primary analysis):
| Key efficacy endpoints | Atezolizumab + Bevacizumab | Sorafenib |
|---|---|---|
| OS | n=336 | n=165 |
| No. of deaths (%) | 96 (28.6%) | 65 (39.4%) |
| Median time to event (months) | NE | 13.2 |
| 95% CI | (NE, NE) | (10.4, NE) |
| Stratified hazard ratio‡ (95% CI) | 0.58 (0.42, 0.79) | |
| p-value1 | 0.0006 | |
| 6-month OS (%) | 84.8% | 72.3% |
| IRF-assessed PFS, RECIST 1.1 | n=336 | n=165 |
| No. of events (%) | 197 (58.6%) | 109 (66.1%) |
| Median duration of PFS (months) | 6.8 | 4.3 |
| 95% CI | (5.8, 8.3) | (4.0, 5.6) |
| Stratified hazard ratio‡ (95% CI) | 0.59 (0.47, 0.76) | |
| p-value1 | <0.0001 | |
| 6-month PFS | 54.5% | 37.2% |
| IRF-assessed ORR, RECIST 1.1 | n=326 | n=159 |
| No. of confirmed responders (%) | 89 (27.3%) | 19 (11.9%) |
| 95% CI | (22.5, 32.5) | (7.4, 18.0) |
| p-value2 | <0.0001 | |
| No. of complete responses (%) | 18 (5.5%) | 0 |
| No. of partial responses (%) | 71 (21.8%) | 19 (11.9%) |
| No. of stable disease (%) | 151 (46.3%) | 69 (43.4%) |
| IRF-assessed DOR, RECIST 1.1 | n=89 | n=19 |
| Median in months | NE | 6.3 |
| 95% CI | (NE, NE) | (4.7, NE) |
| Range (months) | (1.3+, 13.4+) | (1.4+, 9.1+) |
| IRF-assessed ORR, HCC mRECIST | n=325 | n=158 |
| No. of confirmed responders (%) | 108 (33.2%) | 21 (13.3%) |
| 95% CI | (28.1, 38.6) | (8.4, 19.6) |
| p-value2 | <0.0001 | |
| No. of complete responses (%) | 33 (10.2%) | 3 (1.9%) |
| No. of partial responses (%) | 75 (23.1%) | 18 (11.4%) |
| No. of stable disease (%) | 127 (39.1%) | 66 (41.8%) |
| IRF-assessed DOR, HCC mRECIST | n=108 | n=21 |
| Median in months | NE | 6.3 |
| 95% CI | (NE, NE) | (4.9, NE) |
| Range (months) | (1.3+, 13.4+) | (1.4+, 9.1+) |
Figure 21. Kaplan-Meier curve for OS in the ITT population (IMbrave150 updated analysis):
Figure 22. Kaplan-Meier curve for IRF-PFS per RECIST v1.1 in the ITT population (IMbrave150 primary analysis):
Efficacy in elderly
No overall differences in efficacy were observed between patients ≥65 years of age and younger patients receiving atezolizumab monotherapy. In study IMpower150, age ≥65 was associated with a diminished effect of atezolizumab in patients receiving atezolizumab in combination with carboplatin and paclitaxel. In studies IMpower150, IMpower133 and IMpower110, data for patients ≥75 years of age are too limited to draw conclusions on this population.
Paediatric population
An early phase, multi-centre open-label study was conducted in paediatric (<18 years, n=69) and young adult patients (18-30 years, n=18) with relapsed or progressive solid tumours as well as with Hodgkin’s and non-Hodgkin’s lymphoma, to evaluate the safety and pharmacokinetics of atezolizumab. Patients were treated with 15 mg/kg bw atezolizumab intravenously every 3 weeks (see section 5.2).
Pharmacokinetic properties
Exposure to atezolizumab increased dose proportionally over the dose range 1 mg/kg bw to 20 mg/kg bw including the fixed dose 1 200 mg administered every 3 weeks. A population analysis that included 472 patients described atezolizumab pharmacokinetics for the dose range: 1 to 20 mg/kg bw with a linear two-compartment disposition model with first-order elimination. The pharmacokinetic properties of 840 mg intravenous atezolizumab administered every 2 weeks, 1 200 mg administered every 3 weeks, and 1 680 mg administered every 4 weeks are the same; comparable total exposures are expected to be achieved with these three dosing regimens. A population pharmacokinetic analysis suggests that steady-state is obtained after 6 to 9 weeks of multiple dosing. The systemic accumulation in area under the curve, maximum concentration and trough concentration was 1.91, 1.46 and 2.75-fold, respectively.
Absorption
Atezolizumab is administered as an intravenous infusion.
Distribution
A population pharmacokinetic analysis indicates that central compartment volume of distribution is 3.28 L and volume at steady-state is 6.91 L in the typical patient.
Biotransformation
The metabolism of atezolizumab has not been directly studied. Antibodies are cleared principally by catabolism.
Elimination
A population pharmacokinetic analysis indicates that the clearance of atezolizumab is 0.200 L/day and the typical terminal elimination half-life is 27 days.
Special populations
Based on population PK and exposure-response analyses age (21-89 years), region, ethnicity, renal impairment, mild hepatic impairment, level of PD-L1 expression, or ECOG performance status have no effect on atezolizumab pharmacokinetics. Body weight, gender, positive ADA status, albumin levels and tumour burden have a statistically significant, but not clinically relevant effect on atezolizumab pharmacokinetics. No dose adjustments are recommended.
Elderly
No dedicated studies of atezolizumab have been conducted in elderly patients. The effect of age on the pharmacokinetics of atezolizumab was assessed in a population pharmacokinetic analysis. Age was not identified as a significant covariate influencing atezolizumab pharmacokinetics based on patients of age range of 21-89 years (n=472), and median of 62 years of age. No clinically important difference was observed in the pharmacokinetics of atezolizumab among patients <65 years (n=274), patients between 65−75 years (n=152) and patients >75 years (n=46) (see section 4.2).
Paediatric population
The pharmacokinetic results from one early-phase, multi-centre open-label study that was conducted in paediatric (<18 years, n=69) and young adult patients (18-30 years, n=18), show that the clearance and volume of distribution of atezolizumab were comparable between paediatric patients receiving 15 mg/kg bw and young adult patients receiving 1 200 mg of atezolizumab every 3 weeks when normalized by body weight, with exposure trending lower in paediatric patients as body weight decreased. These differences were not associated with a decrease in atezolizumab concentrations below the therapeutic target exposure. Data for children <2 years is limited thus no definitive conclusions can be made.
Renal impairment
No dedicated studies of atezolizumab have been conducted in patients with renal impairment. In the population pharmacokinetic analysis, no clinically important differences in the clearance of atezolizumab were found in patients with mild (estimated glomerular filtration rate [eGFR] 60 to 89 mL/min/1.73 m²; n=208) or, moderate (eGFR 30 to 59 mL/min/1.73 m²; n=116) renal impairment compared to patients with normal (eGFR greater than or equal to 90 mL/min/1.73 m²; n=140) renal function. Only a few patients had severe renal impairment (eGFR 15 to 29 mL/min/1.73 m²; n=8) (see section 4.2). The effect of severe renal impairment on the pharmacokinetics of atezolizumab is unknown.
Hepatic impairment
No dedicated studies of atezolizumab have been conducted in patients with hepatic impairment. In the population pharmacokinetic analysis, there were no clinically important differences in the clearance of atezolizumab observed in patients with mild hepatic impairment (bilirubin ≤ ULN and AST > ULN or bilirubin >1.0 × to 1.5 × ULN and any AST) or moderate hepatic impairment (bilirubin >1.5 to 3x ULN and any AST) in comparison to patients with normal hepatic function (bilirubin ≤ ULN and AST ≤ ULN). No data are available in patients with severe hepatic impairment (bilirubin >3 X ULN and any AST). Hepatic impairment was defined by the National Cancer Institute-Organ Dysfunction Working Group (NCI-ODWG) criteria of hepatic dysfunction (see section 4.2). The effect of severe hepatic impairment (bilirubin >3 × ULN and any AST) on the pharmacokinetics of atezolizumab is unknown.
Preclinical safety data
Carcinogenicity
Carcinogenicity studies have not been performed to establish the carcinogenic potential of atezolizumab.
Mutagenicity
Mutagenicity studies have not been performed to establish the mutagenic potential of atezolizumab. However, monoclonal antibodies are not expected to alter DNA or chromosomes.
Fertility
No fertility studies have been conducted with atezolizumab; however assessment of the cynomolgus monkey male and female reproductive organs was included in the chronic toxicity study. Weekly administration of atezolizumab to female monkeys at an estimated AUC approximately 6 times the AUC in patients receiving the recommended dose caused an irregular menstrual cycle pattern and a lack of newly formed corpora lutea in the ovaries which were reversible. There was no effect on the male reproductive organs.
Teratogenicity
No reproductive or teratogenicity studies in animals have been conducted with atezolizumab. Animal studies have demonstrated that inhibition of the PD-L1/PD-1 pathway can lead to immune-mediated rejection of the developing foetus resulting in foetal death. Administration of atezolizumab could cause foetal harm, including embryo-foetal lethality.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.