TOVANOR BREEZHALER Inhalation powder, hard capsule Ref.[6836] Active ingredients: Glycopyrronium
Source: European Medicines Agency (EU) Revision Year: 2021 Publisher: Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road, Dublin 4, Ireland
Therapeutic indications
Tovanor Breezhaler is indicated as a maintenance bronchodilator treatment to relieve symptoms in adult patients with chronic obstructive pulmonary disease (COPD).
Posology and method of administration
Posology
The recommended dose is the inhalation of the content of one capsule once daily using the Tovanor Breezhaler inhaler.
Tovanor Breezhaler is recommended to be administered, at the same time of the day each day. If a dose is missed, the next dose should be taken as soon as possible. Patients should be instructed not to take more than one dose in a day.
Special populations
Elderly population
Tovanor Breezhaler can be used at the recommended dose in elderly patients (75 years of age and older) (see section 4.8).
Renal impairment
Tovanor Breezhaler can be used at the recommended dose in patients with mild to moderate renal impairment. In patients with severe renal impairment or end-stage renal disease requiring dialysis Tovanor Breezhaler should be used only if the expected benefit outweighs the potential risk since the systemic exposure to glycopyrronium may be increased in this population (see sections 4.4 and 5.2).
Hepatic impairment
No studies have been conducted in patients with hepatic impairment. Glycopyrronium is predominantly cleared by renal excretion and therefore no major increase in exposure is expected in patients with hepatic impairment. No dose adjustment is required in patients with hepatic impairment.
Paediatric population
There is no relevant use of Tovanor Breezhaler in the paediatric population (under 18 years) in the indication COPD.
Method of administration
For inhalation use only.
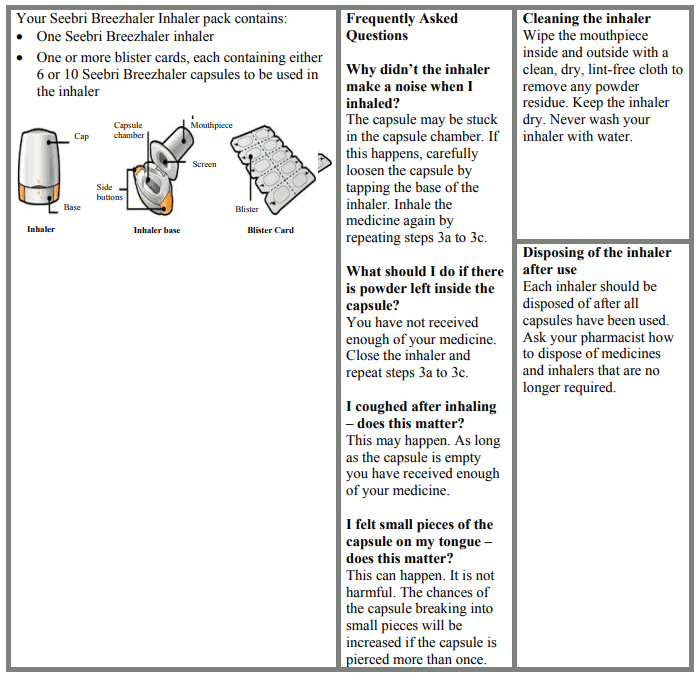
The capsules must be administered only using the Tovanor Breezhaler inhaler (see section 6.6).
The capsules must only be removed from the blister immediately before use.
The capsules must not be swallowed.
Patients should be instructed on how to administer the medicinal product correctly. Patients who do not experience improvement in breathing should be asked if they are swallowing the medicinal product rather than inhaling it.
For instructions on use of the medicinal product before administration, see section 6.6.
Overdose
High doses of glycopyrronium may lead to anticholinergic signs and symptoms for which symptomatic treatment may be indicated.
Acute intoxication by inadvertent oral ingestion of Tovanor Breezhaler capsules is unlikely due to the low oral bioavailability (about 5%).
Peak plasma levels and total systemic exposure following intravenous administration of 150 micrograms glycopyrronium bromide (equivalent to 120 micrograms glycopyrronium) in healthy volunteers were respectively about 50-fold and 6-fold higher than the peak and total exposure at steady-state achieved with the recommended dose (44 micrograms once daily) of Tovanor Breezhaler and were well tolerated.
Shelf life
2 years.
Each inhaler should be disposed of after all capsules have been used.
Special precautions for storage
Do not store above 25°C.
The capsules must always be stored in the original blister in order to protect from moisture. The capsules must only be removed immediately before use.
Nature and contents of container
Tovanor Breezhaler is a single-dose inhaler. Inhaler body and cap are made from acrylonitrile butadiene styrene, push buttons are made from methyl metacrylate acrylonitrile butadiene styrene. Needles and springs are made from stainless steel. Each blister strip contains either 6 or 10 hard capsules.
PA/Alu/PVC – Alu perforated unit-dose blister
Packs containing 6x1, 10x1, 12x1 or 30x1 hard capsules, together with one inhaler.
Multipacks containing 90 (3 packs of 30x1) hard capsules and 3 inhalers.
Multipacks containing 96 (4 packs of 24x1) hard capsules and 4 inhalers.
Multipacks containing 150 (15 packs of 10x1) hard capsules and 15 inhalers.
Multipacks containing 150 (25 packs of 6x1) hard capsules and 25 inhalers.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
The inhaler provided with each new prescription should be used. Each inhaler should be disposed of after all capsules have been used.
Instructions for handling and use
Please read the full Instructions for Use before using the Tovanor Breezhaler.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.