TRELSTAR Suspension for injection Ref.[109541] Active ingredients: Triptorelin
Source: FDA, National Drug Code (US) Revision Year: 2023
1. Indications and Usage
TRELSTAR is indicated for the treatment of advanced prostate cancer [see Clinical Studies (14)].
2. Dosage and Administration
2.1 Dosing Information
TRELSTAR must be administered under the supervision of a physician.
TRELSTAR is administered by a single intramuscular injection in either buttock. Dosing schedule depends on the product strength selected (Table 1). The lyophilized microgranules are to be reconstituted in sterile water. No other diluent should be used.
Table 1. TRELSTAR Recommended Dosing:
| Dosage | 3.75 mg | 11.25 mg | 22.5 mg |
| Recommended dose | 1 injection every 4 weeks | 1 injection every 12 weeks | 1 injection every 24 weeks |
Due to different release characteristics, the dosage strengths are not additive and must be selected based upon the desired dosing schedule.
The suspension should be administered immediately after reconstitution.
As with other drugs administered by intramuscular injection, the injection site should be alternated periodically.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
2.2 Reconstitution Instructions for TRELSTAR with MIXJECT SYSTEM
Important: Please read the instructions completely and prepare the patient before you begin the MIXJECT activation and drug administration procedure.
MIXJECT Preparation and Activation
Wash your hands with soap and hot water and put on gloves immediately prior to preparing the injection. Place the sealed tray on a clean, flat surface that is covered with a sterile pad or cloth. Peel the cover away from the tray and remove the MIXJECT components and the TRELSTAR vial.
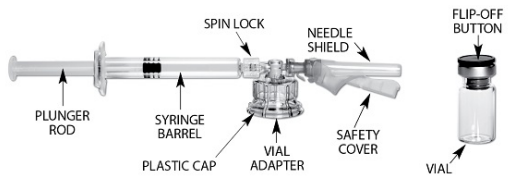
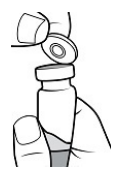 | STEP 1 – PREPARE VIAL Remove the flip-off button cap from the vial, revealing the rubber stopper. Place the vial in a standing upright position on the prepared surface. Disinfect the rubber stopper with the alcohol wipe. Discard the alcohol wipe and allow the stopper to dry. |
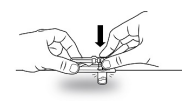 | STEP 2 – APPLY MIXJECT VIAL ADAPTERPeel the cover away from the blister pack containing the vial adapter. Do not remove the vial adapter from the blister pack. On a level surface, place the blister pack containing the vial adapter firmly on the vial top. Ensure the spike is centered and vertical when piercing the vial. Push down gently until you feel it snap into place. Remove the blister pack from the vial adapter. Ensure luer lock for needle connection is tight. |
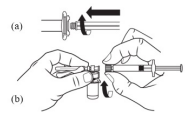 | STEP 3 – PREPARE SYRINGE BARREL(a) Screw the plunger rod into the gray stopper in the barrel end of the syringe. Grasp the plastic ‘spin lock’ collar on the syringe barrel with index finger and thumb, unscrew and discard the gray rubber cap from the syringe barrel. (b) Maintain your grip on the spin lock, ensuring that you have clear visibility of the connection. Attach the syringe to the vial adapter by screwing the spin lock clockwise into the opening on the side of the vial adapter. Gently twist the syringe until it stops turning to ensure a tight connection. (Overtightening can result in a poor connection and leakage) |
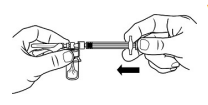 | STEP 4 – TRANSFER DILUENT TO VIALWhile keeping the syringe and vial securely coupled in an upright position, slowly push the plunger to transfer all of the diluent into the vial. Ensure that diluent rinses the sides of the vial. Keep the plunger rod depressed and do not release the plunger rod. |
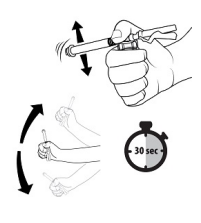 | STEP 5 – MIX TRELSTAR SUSPENSIONKeep the plunger rod depressed, grip the vial and vial adapter firmly and shake vigorously for 30 seconds to mix the contents thoroughly. This will ensure complete mixing of TRELSTAR and the sterile water diluent. The suspension should appear homogeneous and milky. In order to avoid separation of the suspension, proceed to the next steps without delay. The product must be injected within less than 2 minutes from reconstitution. Note: If there is sedimentation in the vial, shake again. |
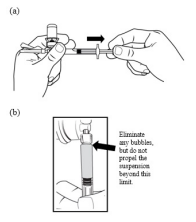 | STEP 6 – LOAD THE SYRINGE WITH TRELSTAR(a) Invert the MIXJECT system so that the vial is at the top. Hold the MIXJECT system firmly by the luer lock connection and syringe barrel and pull back the plunger rod slowly to draw the reconstituted TRELSTAR into the syringe while maintaining pressure on the plunger. (b) Rotate MIXJECT system so syringe is vertical. Remove air bubbles by expelling air into the vial but do not propel the suspension beyond the luer lock. |
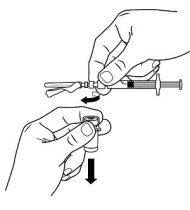 | STEP 7 – DISCONNECT VIAL ADAPTERReturn the vial to its upright position. Hold the barrel and luer lock firmly. With your other hand, disconnect the vial adapter and vial from the MIXJECT syringe by grasping the plastic cap of the vial adapter and turning it clockwise. Grasp only the vial adapter cap when removing. |
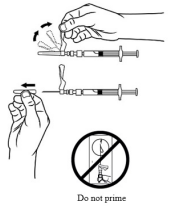 | STEP 8 – PREPARE NEEDLE FOR INJECTION Make sure that the patient is ready for the administration. Just prior to administration, perform at least 5 inversions with the syringe to resuspend the particles. Lift up the safety cover and remove the clear plastic needle shield by pulling it from the assembly. The safety cover should be perpendicular to the needle, with the needle facing away from you. Do not prime the needle. The syringe containing the TRELSTAR suspension is now ready for administration. The suspension should be administered immediately (less than 2 minutes) after reconstitution to avoid excessive thickening of the suspension. |
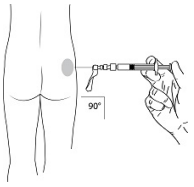 | STEP 9 – ADMINISTRATION Administer the injection by inserting the needle at a 90-degree angle into the large gluteal muscle. Ensure that the full amount of the product is injected within 10 seconds without interruption. Injection sites should be alternated. |
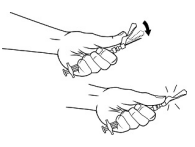 | STEP 10 – SAFETY LOCK AFTER INJECTIONAfter administering the injection, immediately activate the safety mechanism by centering your thumb or forefinger on the textured finger pad area of the safety cover and pushing it forward over the needle until you hear or feel it lock in place. Use the one-handed technique and activate the mechanism away from yourself and others. Immediately discard the syringe into a sharps container after a single use. |
10. Overdosage
There is no experience of overdosage in clinical trials. In single dose toxicity studies in mice and rats, the subcutaneous LD50 of triptorelin was 400 mg/kg in mice and 250 mg/kg in rats, approximately 500 and 600 times, respectively, the estimated monthly human dose based on body surface area. If overdosage occurs, therapy should be discontinued immediately and the appropriate supportive and symptomatic treatment administered.
16.2. Storage and Handling
Store at 20-25°C (68-77°F). [See USP Controlled Room Temperature.] Do not freeze TRELSTAR with MIXJECT.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.