TRUXIMA Concentrate for solution for infusion Ref.[7416] Active ingredients: Rituximab
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Celltrion Healthcare Hungary Kft., 1062 Budapest, Váci út 1-3. WestEnd Office Building B torony, Hungary
Pharmacodynamic properties
Pharmacotherapeutic group: antineoplastic agents, monoclonal antibodies
ATC code: L01FA01
Truxima is a biosimilar medicinal product.
Rituximab binds specifically to the transmembrane antigen, CD20, a non-glycosylated phosphoprotein, located on pre-B and mature B lymphocytes. The antigen is expressed on >95% of all B cell non-Hodgkin's lymphomas.
CD20 is found on both normal and malignant B cells, but not on haematopoietic stem cells, pro-B cells, normal plasma cells or other normal tissue. This antigen does not internalize upon antibody binding and is not shed from the cell surface. CD20 does not circulate in the plasma as a free antigen and, thus, does not compete for antibody binding.
The Fab domain of rituximab binds to the CD20 antigen on B lymphocytes and the Fc domain can recruit immune effector functions to mediate B cell lysis. Possible mechanisms of effector-mediated cell lysis include complement-dependent cytotoxicity (CDC) resulting from C1q binding, and antibody-dependent cellular cytotoxicity (ADCC) mediated by one or more of the Fcγ receptors on the surface of granulocytes, macrophages and NK cells. Rituximab binding to CD20 antigen on B lymphocytes has also been demonstrated to induce cell death via apoptosis.
Peripheral B cell counts declined below normal following completion of the first dose of rituximab. In patients treated for haematological malignancies, B cell recovery began within 6 months of treatment and generally returned to normal levels within 12 months after completion of therapy, although in some patients this may take longer (up to a median recovery time of 23 months post-induction therapy). In rheumatoid arthritis patients, immediate depletion of B cells in the peripheral blood was observed following two infusions of 1000 mg rituximab separated by a 14-day interval. Peripheral blood B cell counts begin to increase from week 24 and evidence for repopulation is observed in the majority of patients by week 40, whether rituximab was administered as monotherapy or in combination with methotrexate. A small proportion of patients had prolonged peripheral B cell depletion lasting 2 years or more after their last dose of rituximab. In patients with GPA or MPA, the number of peripheral blood B cells decreased to <10 cells/μL after two weekly infusions of rituximab 375 mg/m², and remained at that level in most patients up to the 6 month time point. The majority of patients (81%) showed signs of B cell return, with counts >10 cells/μL by month 12, increasing to 87% of patients by month 18.
Clinical experience in non-Hodgkin's lymphoma and in chronic lymphocytic leukaemia
Follicular lymphoma
Monotherapy
Initial treatment, weekly for 4 doses:
In the pivotal trial, 166 patients with relapsed or chemoresistant low-grade or follicular B cell NHL received 375 mg/m² of rituximab as an intravenous infusion once weekly for four weeks. The overall response rate (ORR) in the intent-to-treat (ITT) population was 48% (CI95% 41% - 56%) with a 6% complete response (CR) and a 42% partial response (PR) rate. The projected median time to progression (TTP) for responding patients was 13.0 months. In a subgroup analysis, the ORR was higher in patients with IWF B, C, and D histological subtypes as compared to IWF A subtype (58% vs. 12%), higher in patients whose largest lesion was <5 cm vs. >7 cm in greatest diameter (53% vs. 38%), and higher in patients with chemosensitive relapse as compared to chemoresistant (defined as duration of response <3 months) relapse (50% vs. 22%). ORR in patients previously treated with autologous bone marrow transplant (ABMT) was 78% versus 43% in patients with no ABMT. Neither age, sex, lymphoma grade, initial diagnosis, presence nor absence of bulky disease, normal or high LDH nor presence of extranodal disease had a statistically significant effect (Fisher's exact test) on response to rituximab. A statistically significant correlation was noted between response rates and bone marrow involvement. 40% of patients with bone marrow involvement responded compared to 59% of patients with no bone marrow involvement (p=0.0186). This finding was not supported by a stepwise logistic regression analysis in which the following factors were identified as prognostic factors: histological type, bcl-2 positivity at baseline, resistance to last chemotherapy and bulky disease.
Initial treatment, weekly for 8 doses:
In a multicentre, single-arm trial, 37 patients with relapsed or chemoresistant, low grade or follicular B cell NHL received 375 mg/m² of rituximab as intravenous infusion weekly for eight doses. The ORR was 57% (95% Confidence interval (CI); 41% – 73%; CR 14%, PR 43%) with a projected median TTP for responding patients of 19.4 months (range 5.3 to 38.9 months).
Initial treatment, bulky disease, weekly for 4 doses:
In pooled data from three trials, 39 patients with relapsed or chemoresistant, bulky disease (single lesion ≥10 cm in diameter), low grade or follicular B cell NHL received 375 mg/m² of rituximab as intravenous infusion weekly for four doses. The ORR was 36% (CI95% 21% – 51%; CR 3%, PR 33%) with a median TTP for responding patients of 9.6 months (range 4.5 to 26.8 months).
Re-treatment, weekly for 4 doses:
In a multicentre, single-arm trial, 58 patients with relapsed or chemoresistant low grade or follicular B cell NHL, who had achieved an objective clinical response to a prior course of rituximab, were re-treated with 375 mg/m² of rituximab as intravenous infusion weekly for four doses. Three of the patients had received two courses of rituximab before enrolment and thus were given a third course in the study. Two patients were re-treated twice in the study. For the 60 re-treatments on study, the ORR was 38% (CI95% 26% – 51%; 10% CR, 28% PR) with a projected median TTP for responding patients of 17.8 months (range 5.4 – 26.6). This compares favourably with the TTP achieved after the prior course of rituximab (12.4 months).
Initial treatment, in combination with chemotherapy
In an open-label randomised trial, a total of 322 previously untreated patients with follicular lymphoma were randomised to receive either CVP chemotherapy (cyclophosphamide 750 mg/m², vincristine 1.4 mg/m² up to a maximum of 2 mg on day 1, and prednisolone 40 mg/m²/day on days 1- 5) every 3 weeks for 8 cycles or rituximab 375 mg/m² in combination with CVP (R-CVP). Rituximab was administered on the first day of each treatment cycle. A total of 321 patients (162 R-CVP, 159 CVP) received therapy and were analysed for efficacy. The median follow-up of patients was 53 months. R-CVP led to a significant benefit over CVP for the primary endpoint, time to treatment failure (27 months vs. 6.6 months, p<0.0001, log-rank test). The proportion of patients with a tumour response (CR, CRu, PR) was significantly higher (p<0.0001 Chi-Square test) in the R-CVP group (80.9%) than the CVP group (57.2%). Treatment with R-CVP significantly prolonged the time to disease progression or death compared to CVP, 33.6 months and 14.7 months, respectively (p<0.0001, log-rank test). The median duration of response was 37.7 months in the R-CVP group and was 13.5 months in the CVP group (p<0.0001, log-rank test).
The difference between the treatment groups with respect to overall survival showed a significant clinical difference (p=0.029, log-rank test stratified by centre): survival rates at 53 months were 80.9% for patients in the R-CVP group compared to 71.1% for patients in the CVP group.
Results from three other randomised trials using rituximab in combination with chemotherapy regimen other than CVP (CHOP, MCP, CHVP/Interferon-α) have also demonstrated significant improvements in response rates, time-dependent parameters as well as in overall survival. Key results from all four studies are summarised in Table 8.
Table 8. Summary of key results from four phase III randomised studies evaluating the benefit of rituximab with different chemotherapy regimens in follicular lymphoma:
| Study | Treatment, N | Median FU, months | ORR, % | CR, % | Median TTF/PFS/EFS months | OS rates, % |
|---|---|---|---|---|---|---|
| M39021 | CVP, 159 R-CVP, 162 | 53 | 57 81 | 10 41 | Median TTP: 14.7 33.6 P<0.0001 | 53-months 71.1 80.9 p=0.029 |
| GLSG'00 | CHOP, 205 R-CHOP, 223 | 18 | 90 96 | 17 20 | Median TTF: 2.6 years Not reached p<0.001 | 18-months 90 95 p=0.016 |
| OSHO‐39 | MCP, 96 R-MCP, 105 | 47 | 75 92 | 25 50 | Median PFS: 28.8 Not reached p<0.0001 | 48-months 74 87 p=0.0096 |
| FL2000 | CHVP-IFN, 183 R-CHVP-IFN, 175 | 42 | 85 94 | 49 76 | Median EFS: 36 Not reached p<0.0001 | 42-months 84 91 p=0.029 |
EFS – Event Free Survival
TTP – Time to progression or death
PFS – Progression-Free Survival
TTF – Time to Treatment Failure
OS rates – survival rates at the time of the analyses
Maintenance therapy
Previously untreated follicular lymphoma:
In a prospective, open label, international, multicentre, phase III trial 1193 patients with previously untreated advanced follicular lymphoma received induction therapy with R-CHOP (n=881), R-CVP (n=268) or R-FCM (n=44), according to the investigators' choice. A total of 1078 patients responded to induction therapy, of which 1018 were randomised to rituximab maintenance therapy (n=505) or observation (n=513). The two treatment groups were well balanced with regards to baseline characteristics and disease status. Rituximab maintenance treatment consisted of a single infusion of rituximab at 375 mg/m² body surface area given every 2 months until disease progression or for a maximum period of two years.
The pre-specified primary analysis was conducted at a median observation time of 25 months from randomisation, maintenance therapy with rituximab resulted in a clinically relevant and statistically significant improvement in the primary endpoint of investigator assessed progression-free survival (PFS) as compared to observation in patients with previously untreated follicular lymphoma (Table 9).
Significant benefit from maintenance treatment with rituximab was also seen for the secondary endpoints event-free survival (EFS), time to next anti-lymphoma treatment (TNLT) time to next chemotherapy (TNCT) and overall response rate (ORR) in the primary analysis (Table 9).
Data from extended follow-up of patients in the study (median follow-up 9 years) confirmed the long- term benefit of rituximab maintenance therapy in terms of PFS, EFS, TNLT and TNCT (Table 9).
Table 9. Overview of efficacy results for rituximab maintenance vs. observation at the protocol-defined primary analysis and after 9 years median follow-up (final analysis):
| Primary analysis (median FU: 25 months) | Final analysis (median FU: 9.0 years) | |||
|---|---|---|---|---|
| Observation N=513 | Rituximab N=505 | Observation N=513 | Rituximab N=505 | |
| Primary efficacy | ||||
| Progression-free survival (median) | NR | NR | 4.06 years | 10.49 years |
| log-rank p value | <0.0001 | <0.0001 | ||
| hazard ratio (95% CI) risk reduction | 0.50 (0.39, 0.64) 50% | 0.61 (0.52, 0.73) 39% | ||
| Secondary efficacy | ||||
| Overall survival (median) | NR | NR | NR | NR |
| log-rank p value | 0.7246 | 0.7948 | ||
| hazard ratio (95% CI) risk reduction | 0.89 (0.45, 1.74) 11% | 1.04 (0.77, 1.40) -6% | ||
| Event-free survival (median) | 38 months | NR | 4.04 years | 9.25 years |
| log-rank p value | <0.0001 | <0.0001 | ||
| hazard ratio (95% CI) risk reduction | 0.54 (0.43, 0.69) 46% | 0.64 (0.54, 0.76) 36% | ||
| TNLT (median) | NR | NR | 6.11 years | NR |
| log-rank p value | 0.0003 | <0.0001 | ||
| hazard ratio (95% CI) risk reduction | 0.61 (0.46, 0.80) 39% | 0.66 (0.55, 0.78) 34% | ||
| TNCT (median) | NR | NR | 9.32 years | NR |
| log-rank p value | 0.0011 | 0.0004 | ||
| hazard ratio (95% CI) risk reduction | 0.60 (0.44, 0.82) 40% | 0.71 (0.59, 0.86) 39% | ||
| Overall response rate* | 55% | 74% | 61% | 79% |
| chi-squared test p value | <0.0001 | <0.0001 | ||
| odds ratio (95% CI) | 2.33 (1.73, 3.15) | 2.43 (1.84, 3.22) | ||
| Complete response (CR/CRu) rate* | 48% | 67% | 53% | 67% |
| chi-squared test p value | <0.0001 | <0.0001 | ||
| odds ratio (95% CI) | 2.21 (1.65, 2.94) | 2.34 (1.80, 3.03) | ||
* at end of maintenance/observation; final analysis results based on median follow-up of 73 months.
CI: confidence interval; FU: follow-up; OS: overall survival; TNLT: time to next anti-lymphoma treatment; TNCT: time to next chemotherapy treatment; NR: not reached at time of clinical cut-off.
Rituximab maintenance treatment provided consistent benefit in all predefined subgroups tested: gender (male, female), age (<60 years, ≥60 years), FLIPI score (≤1, 2 or ≥3), induction therapy (R-CHOP, R-CVP or R-FCM) and regardless of the quality of response to induction treatment (CR, CRu or PR). Exploratory analyses of the benefit of maintenance treatment showed a less pronounced effect in elderly patients (>70 years of age), however sample sizes were small.
Relapsed/Refractory follicular lymphoma:
In a prospective, open label, international, multicentre, phase III trial, 465 patients with relapsed/refractory follicular lymphoma were randomised in a first step to induction therapy with either CHOP (cyclophosphamide, doxorubicin, vincristine, prednisolone; n=231) or rituximab plus CHOP (R-CHOP, n=234). The two treatment groups were well balanced with regard to baseline characteristics and disease status. A total of 334 patients achieving a complete or partial remission following induction therapy were randomised in a second step to rituximab maintenance therapy (n=167) or observation (n=167). Rituximab maintenance treatment consisted of a single infusion of rituximab at 375 mg/m² body surface area given every 3 months until disease progression or for a maximum period of two years.
The final efficacy analysis included all patients randomised to both parts of the study. After a median observation time of 31 months for patients randomised to the induction phase, R-CHOP significantly improved the outcome of patients with relapsed/refractory follicular lymphoma when compared to CHOP (see Table 10).
Table 10. Induction phase: overview of efficacy results for CHOP vs. R-CHOP (31 months median observation time):
| CHOP | R‐CHOP | p‐value | Risk Reduction1 | |
|---|---|---|---|---|
| Primary efficacy | ||||
| ORR2 | 74% | 87% | 0.0003 | NA |
| CR2 | 16% | 29% | 0.0005 | NA |
| PR2 | 58% | 58% | 0.9449 | NA |
1 Estimates were calculated by hazard ratios
2 Last tumour response as assessed by the investigator. The "primary" statistical test for "response" was the trend test of CR versus PR versus non-response (p<0.0001)
Abbreviations: NA, not available; ORR: overall response rate; CR: complete response; PR: partial response
For patients randomised to the maintenance phase of the trial, the median observation time was 28 months from maintenance randomisation. Maintenance treatment with rituximab led to a clinically relevant and statistically significant improvement in the primary endpoint, PFS, (time from maintenance randomisation to relapse, disease progression or death) when compared to observation alone (p<0.0001 log-rank test). The median PFS was 42.2 months in the rituximab maintenance arm compared to 14.3 months in the observation arm. Using a cox regression analysis, the risk of experiencing progressive disease or death was reduced by 61% with rituximab maintenance treatment when compared to observation (95% CI; 45%-72%). Kaplan-Meier estimated progression-free rates at 12 months were 78% in the rituximab maintenance group vs. 57% in the observation group. An analysis of overall survival confirmed the significant benefit of rituximab maintenance over observation (p=0.0039 log-rank test). Rituximab maintenance treatment reduced the risk of death by 56% (95% CI; 22%-75%).
Table 11. Maintenance phase: overview of efficacy results rituximab vs. observation (28 months median observation time):
| Efficacy parameter | Kaplan-Meier estimate of median time to event (months) | Risk reduction | ||
|---|---|---|---|---|
| Observation (N=167) | Rituximab (N=167) | Log-rank p value | ||
| Progression‐free survival (PFS) | 14.3 | 42.2 | <0.0001 | 61% |
| Overall survival | NR | NR | 0.0039 | 56% |
| Time to new lymphoma treatment | 20.1 | 38.8 | <0.0001 | 50% |
| Disease‐free survivala | 16.5 | 53.7 | 0.0003 | 67% |
| Subgroup analysis | ||||
| PFS | ||||
| CHOP | 11.6 | 37.5 | <0.0001 | 71% |
| R-CHOP | 22.1 | 51.9 | 0.0071 | 46% |
| CR | 14.3 | 52.8 | 0.0008 | 64% |
| PR | 14.3 | 37.8 | <0.0001 | 54% |
| OS | ||||
| CHOP | NR | NR | 0.0348 | 55% |
| R-CHOP | NR | NR | 0.0482 | 56% |
NR: not reached; a: only applicable to patients achieving a C
The benefit of rituximab maintenance treatment was confirmed in all subgroups analysed, regardless of induction regimen (CHOP or R-CHOP) or quality of response to induction treatment (CR or PR) (Table 11). Rituximab maintenance treatment significantly prolonged median PFS in patients responding to CHOP induction therapy (median PFS 37.5 months vs. 11.6 months, p< 0.0001) as well as in those responding to R-CHOP induction (median PFS 51.9 months vs. 22.1 months, p=0.0071). Although subgroups were small, rituximab maintenance treatment provided a significant benefit in terms of overall survival for both patients responding to CHOP and patients responding to R-CHOP, although longer follow-up is required to confirm this observation.
Adult Diffuse large B cell non-Hodgkin's lymphoma
In a randomised, open-label trial, a total of 399 previously untreated elderly patients (age 60 to 80 years) with diffuse large B cell lymphoma received standard CHOP chemotherapy (cyclophosphamide 750 mg/m², doxorubicin 50 mg/m², vincristine 1.4 mg/m² up to a maximum of 2 mg on day 1, and prednisolone 40 mg/m²/day on days 1-5) every 3 weeks for eight cycles, or rituximab 375 mg/m² plus CHOP (R-CHOP). Rituximab was administered on the first day of the treatment cycle.
The final efficacy analysis included all randomised patients (197 CHOP, 202 R-CHOP), and had a median follow-up duration of approximately 31 months. The two treatment groups were well balanced in baseline disease characteristics and disease status. The final analysis confirmed that R-CHOP treatment was associated with a clinically relevant and statistically significant improvement in the duration of event-free survival (the primary efficacy parameter; where events were death, relapse or progression of lymphoma, or institution of a new anti-lymphoma treatment) (p=0.0001). Kaplan Meier estimates of the median duration of event-free survival were 35 months in the R-CHOP arm compared to 13 months in the CHOP arm, representing a risk reduction of 41%. At 24 months, estimates for overall survival were 68.2% in the R-CHOP arm compared to 57.4% in the CHOP arm. A subsequent analysis of the duration of overall survival, carried out with a median follow-up duration of 60 months, confirmed the benefit of R-CHOP over CHOP treatment (p=0.0071), representing a risk reduction of 32%.
The analysis of all secondary parameters (response rates, progression-free survival, disease-free survival, duration of response) verified the treatment effect of R-CHOP compared to CHOP. The complete response rate after cycle 8 was 76.2% in the R-CHOP group and 62.4% in the CHOP group (p=0.0028). The risk of disease progression was reduced by 46% and the risk of relapse by 51%. In all patient subgroups (gender, age, age adjusted IPI, Ann Arbor stage, ECOG, β2 microglobulin, LDH, albumin, B symptoms, bulky disease, extranodal sites, bone marrow involvement), the risk ratios for event-free survival and overall survival (R-CHOP compared with CHOP) were less than 0.83 and 0.95 respectively. R-CHOP was associated with improvements in outcome for both high- and low-risk patients according to age adjusted IPI.
Clinical laboratory findings
Of 67 patients evaluated for human anti-mouse antibody (HAMA), no responses were noted. Of 356 patients evaluated for anti-drug antibody (ADA), 1.1% (4 patients) were positive.
Chronic lymphocytic leukaemia
In two open-label randomised trials, a total of 817 previously untreated patients and 552 patients with relapsed/refractory CLL were randomised to receive either FC chemotherapy (fludarabine 25 mg/m², cyclophosphamide 250 mg/m², days 1-3) every 4 weeks for 6 cycles or rituximab in combination with FC (R-FC). Rituximab was administered at a dosage of 375 mg/m² during the first cycle one day prior to chemotherapy and at a dosage of 500 mg/m² on day 1 of each subsequent treatment cycle. Patients were excluded from the study in relapsed/refractory CLL if they had previously been treated with monoclonal antibodies or if they were refractory (defined as failure to achieve a partial remission for at least 6 months) to fludarabine or any nucleoside analogue. A total of 810 patients (403 R-FC, 407 FC) for the first-line study (Table 12a and Table 12b) and 552 patients (276 R-FC, 276 FC) for the relapsed/refractory study (Table 13) were analysed for efficacy.
In the first-line study, after a median observation time of 48.1 months, the median PFS was 55 months in the R-FC group and 33 months in the FC group (p < 0.0001, log-rank test). The analysis of overall survival showed a significant benefit of R-FC treatment over FC chemotherapy alone (p=0.0319, log-rank test) (Table 12a). The benefit in terms of PFS was consistently observed in most patient subgroups analysed according to disease risk at baseline (i.e. Binet stages A-C) (Table 12b).
Table 12a. First-line treatment of chronic lymphocytic leukaemia overview of efficacy results for rituximab plus FC vs. FC alone - 48.1 months median observation time:
| Efficacy parameter | Kaplan-Meier estimate of median time to event (months) | Risk reduction | ||
|---|---|---|---|---|
| FC (N=409) | R-FC (N=408) | Log-rank p value | ||
| Progression‐free survival (PFS) | 32.8 | 55.3 | <0.0001 | 45% |
| Overall survival | NR | NR | 0.0319 | 27% |
| Event free survival | 31.3 | 51.8 | <0.0001 | 44% |
| Response rate (CR, nPR, or PR) CR rates | 72.6% 16.9% | 85.8% 36.0% | <0.0001 <0.0001 | N/A N/A |
| Duration of response* | 36.2 | 57.3 | <0.0001 | 44% |
| Disease free survival (DFS)** | 48.9 | 60.3 | 0.0520 | 31% |
| Time to new treatment | 47.2 | 69.7 | <0.0001 | 42% |
Response rate and CR rates analysed using Chi-squared Test. NR: not reached;
N/A: not applicable
* only applicable to patients achieving a CR, nPR, PR
** only applicable to patients achieving a CR
Table 12b. First-line treatment of chronic lymphocytic leukaemia hazard ratios of progression-free survival according to binet stage (ITT) - 48.1 months median observation time:
| Progression‐free survival (PFS) | Number of patients | Hazard ratio (95% CI) | p-value (Wald test, not adjusted) | |
|---|---|---|---|---|
| FC | R‐FC | |||
| Binet stage A | 22 | 18 | 0.39 (0.15; 0.98) | 0.0442 |
| Binet stage B | 259 | 263 | 0.52 (0.41; 0.66) | <0.0001 |
| Binet stage C | 126 | 126 | 0.68 (0.49; 0.95) | 0.0224 |
CI: Confidence Interval
In the relapsed/refractory study, the median progression-free survival (primary endpoint) was 30.6 months in the R-FC group and 20.6 months in the FC group (p=0.0002, log-rank test). The benefit in terms of PFS was observed in almost all patient subgroups analysed according to disease risk at baseline. A slight but 38 not significant improvement in overall survival was reported in the R-FC compared to the FC arm.
Table 13. Treatment of relapsed/refractory chronic lymphocytic leukaemia - overview of efficacy results for rituximab plus FC vs. FC alone (25.3 months median observation time):
| Efficacy parameter | Kaplan-Meier estimate of median time to event (months) | Risk reduction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FC (N=276) | R-FC (N=276) | Log-rank p value | |||||||
| Progression-free survival (PFS) | 20.6 | 30.6 | 0.0002 | 35% | |||||
| Overall survival | 51.9 | NR | 0.2874 | 17% | |||||
| Event free survival | 19.3 | 28.7 | 0.0002 | 36% | |||||
| Response rate (CR, nPR, or PR) | 58.0% | 69.9% | 0.0034 | N/A | CR rates | 13.0% | 24.3% | 0.0007 | N/A |
| Duration of response* | 27.6 | 39.6 | 0.0252 | 31% | |||||
| Disease free survival (DFS)** | 42.2 | 39.6 | 0.8842 | ‐6% | |||||
| Time to new CLL treatment | 34.2 | NR | 0.0024 | 35% | |||||
Response rate and CR rates analysed using Chi-squared Test. NR: not reached; N/A: not applicable
* only applicable to patients achieving a CR, nPR, PR;
** only applicable to patients achieving a CR
Results from other supportive studies using rituximab in combination with other chemotherapy regimens (including CHOP, FCM, PC, PCM, bendamustine and cladribine) for the treatment of previously untreated and/or relapsed/refractory CLL patients have also demonstrated high overall response rates with benefit in terms of PFS rates, albeit with modestly higher toxicity (especially myelotoxicity). These studies support the use of rituximab with any chemotherapy.
Data in approximately 180 patients pre-treated with rituximab have demonstrated clinical benefit (including CR) and are supportive for rituximab re-treatment.
Paediatric population
A multicenter, open-label, randomized study of Lymphome Malin B (LMB) chemotherapy (corticosteroids, vincristine, cyclophosphamide, high-dose methotrexate, cytarabine, doxorubicin, etoposide and triple drug [methotrexate/cytarabine/ corticosteroid] intrathecal therapy) alone or in combination with rituximab was conducted in paediatric patients with previously untreated advanced stage CD20 positive DLBCL/BL/BAL/BLL. Advanced stage is defined as Stage III with elevated LDH level ("B-high"), [LDH > twice the institutional upper limit of the adult normal values (> Nx2)] or any stage IV or BAL. Patients were randomized to receive either LMB chemotherapy or six intravenous infusions of rituximab at a dose of 375mg/m² BSA in combination with LMB chemotherapy (two during each of the two induction courses and one during each of the two consolidation courses) as per the LMB scheme. A total of 328 randomized patients were included in the efficacy analyses, of which one patient under 3 years of age received rituximab in combination with LMB chemotherapy.
The two treatment arms, LMB (LMB chemotherapy) and R-LMB (LMB chemotherapy with rituximab), were well balanced with regards to baseline characteristics. Patients had a median age of 7 and 8 years in the LMB arm and R-LMB arm, respectively. Approximately half of patients were in Group B (50.6% in the LMB arm and 49.4% in the R-LMB arm), 39.6% in Group C1 in both arms, and 9.8% and 11.0% were in Group C3 in the LMB and R-LMB arms, respectively. Based on Murphy staging, most patients were either BL stage III (45.7% in the LMB arm and 43.3% in the R-LMB arm) or BAL, CNS negative (21.3% in the LMB arm and 24.4% in the R-LMB arm). Less than half of the patients (45.1% in both arms) had bone marrow involvement, and most patients (72.6% in the LMB arm and 73.2% in the R-LMB arm) had no CNS involvement. The primary efficacy endpoint was EFS, where an event was defined as occurrence of progressive disease, relapse, second malignancy, death from any cause, or non-response as evidenced by detection of viable cells in residue after the second CYVE course, whichever occurs first. The secondary efficacy endpoints were OS and CR (complete remission).
At the pre-specified interim analysis with approximately 1 year of median follow-up, clinically relevant improvement in the primary endpoint of EFS was observed, with 1-year rate estimates of 94.2% (95% CI, 88.5% - 97.2%) in the R-LMB arm vs. 81.5% (95% CI, 73.0% - 87.8%) in the LMB arm, and adjusted Cox HR 0.33 (95% CI, 0.14 – 0.79). Upon IDMC (independent data monitoring committee) recommendation based on this result, the randomization was halted and patients in the LMB arm were allowed to cross over to receive rituximab.
Primary efficacy analyses were performed in 328 randomized patients with a median follow-up of 3.1 years. The results are described in Table 14.
Table 14. Overview of primary efficacy results (ITT population):
| Analysis | LMB (N=164) | R-LMB (N=164) |
|---|---|---|
| EFS | 28 events | 10 events |
| One-sided log-rank test p-value 0.0006 | ||
| Adjusted Cox HR 0.32 (90% CI: 0.17, 0.58) | ||
| 3-year EFS rates | 82.3% (95% CI: 75.7%, 87.5%) | 93.9% (95% CI: 89.1%, 96.7%) |
| OS | 20 deaths | 8 deaths |
| One-sided log-rank test p-value 0.0061 | ||
| Adjusted Cox model HR 0.36 (95% CI: 0.16; 0.81) | ||
| 3-year OS rates | 87.3% (95% CI: 81.2%, 91.6%) | 95.1% (95% CI: 90.5%, 97.5%) |
| CR rate | 93.6% (95% CI: 88.2%; 97.0%) | 94.0% (95% CI: 88.8%, 97.2%) |
The primary efficacy analysis showed an EFS benefit of rituximab addition to LMB chemotherapy over LMB chemotherapy alone, with an EFS HR 0.32 (90% CI 0.17 - 0.58) from a Cox regression analysis adjusting for national group, histology, and therapeutic group. While no major differences in numbers of patients achieving CR was observed between the two treatment groups, the benefit of rituximab addition to LMB chemotherapy was also shown in the secondary endpoint of OS, with the OS HR of 0.36 (95% CI, 0.16 – 0.81).
The European Medicines Agency has waived the obligation to submit the results of studies with rituximab in all subsets of the paediatric population with follicular lymphoma and chronic lymphocytic leukaemia, and in the paediatric population from birth to < 6 months of age in CD20 positive diffuse large B-cell lymphoma. See section 4.2 for information on paediatric use.
Clinical experience in rheumatoid arthritis
The efficacy and safety of rituximab in alleviating the symptoms and signs of rheumatoid arthritis in patients with an inadequate response to TNF-inhibitors was demonstrated in a pivotal randomised, controlled, double-blind, multicentre trial (Trial 1).
Trial 1 evaluated 517 patients that had experienced an inadequate response or intolerance to one or more TNF inhibitor therapies. Eligible patients had active rheumatoid arthritis, diagnosed according to the criteria of the American College of Rheumatology (ACR). Rituximab was administered as two intravenous infusions separated by an interval of 15 days. Patients received 2 x 1000 mg intravenous infusions of rituximab or placebo in combination with MTX. All patients received concomitant 60 mg oral prednisone on days 2-7 and 30 mg on days 8-14 following the first infusion. The primary endpoint was the proportion of patients who achieved an ACR20 response at week 24. Patients were followed beyond week 24 for long-term endpoints, including radiographic assessment at 56 weeks and at 104 weeks. During this time, 81% of patients, from the original placebo group received rituximab between weeks 24 and 56, under an open label extension study protocol.
Trials of rituximab in patients with early arthritis (patients without prior methotrexate treatment and patients with an inadequate response to methotrexate, but not yet treated with TNF-alpha inhibitors) have met their primary endpoints. Rituximab is not indicated for these patients, since the safety data about long-term rituximab treatment are insufficient, in particular concerning the risk of development of malignancies and PML.
Disease activity outcomes
Rituximab in combination with methotrexate significantly increased the proportion of patients achieving at least a 20% improvement in ACR score compared with patients treated with methotrexate alone (Table 15). Across all development studies the treatment benefit was similar in patients independent of age, gender, body surface area, race, number of prior treatments or disease status.
Clinically and statistically significant improvement was also noted on all individual components of the ACR response (tender and swollen joint counts, patient and physician global assessment, disability index scores (HAQ), pain assessment and C-Reactive Proteins (mg/dL).
Table 15. Clinical response outcomes at primary endpoint in Trial 1 (ITT population):
| Outcome† | Placebo+MTX | Rituximab+MTX (2 x 1000 mg) | |
|---|---|---|---|
| Trial 1 | N=201 | N=298 | |
| ACR20 | 36 (18%) | 153 (51%)*** | |
| ACR50 | 11 (5%) | 80 (27%)*** | |
| ACR70 | 3 (1%) | 37 (12%)*** | |
| EULAR Response (Good/Moderate) | 44 (22%) | 193 (65%)*** | |
| Mean change in DAS | ‐0.34 | ‐1.83*** |
† Outcome at 24 weeks
Significant difference from placebo + MTX at the primary time point: ***p≤0.0001
Patients treated with rituximab in combination with methotrexate had a significantly greater reduction in disease activity score (DAS28) than patients treated with methotrexate alone (Table 15). Similarly, a good to moderate European League Against Rheumatism (EULAR) response was achieved by significantly more rituximab treated patients treated with rituximab and methotrexate compared to patients treated with methotrexate alone (Table 15).
Radiographic response
Structural joint damage was assessed radiographically and expressed as change in modified Total Sharp Score (mTSS) and its components, the erosion score and joint space narrowing score.
In Trial 1, conducted in patients with inadequate response or intolerance to one or more TNF inhibitor therapies, receiving rituximab in combination with methotrexate demonstrated significantly less radiographic progression than patients originally receiving methotrexate alone at 56 weeks. Of the patients originally receiving methotrexate alone, 81% received rituximab either as rescue between weeks 16-24 or in the extension trial, before week 56. A higher proportion of patients receiving the original rituximab/MTX treatment also had no erosive progression over 56 weeks (Table 16).
Table 16. Radiographic outcomes at 1 year (mITT population):
| Placebo+MTX | Rituximab+MTX 2 × 1000 mg | |
|---|---|---|
| Trial 1 | (n=184) | (n=273) |
| Mean change from baseline | ||
| Modified total sharp score | 2.30 | 1.01* |
| Erosion score | 1.32 | 0.60* |
| Joint space narrowing score | 0.98 | 0.41** |
| Proportion of patients with no radiographic change | 46% | 53%, NS |
| Proportion of patients with no erosive change | 52% | 60%, NS |
150 patients originally randomised to placebo + MTX in Trial 1 received at least one course of RTX + MTX by one year
* p<0.05, **p<0.001. Abbreviation: NS: non significant
Inhibition of the rate of progressive joint damage was also observed long term. Radiographic analysis at 2 years in Trial 1 demonstrated significantly reduced progression of structural joint damage in patients receiving rituximab in combination with methotrexate compared to methotrexate alone as well as a significantly higher proportion of patients with no progression of joint damage over the 2-year period.
Physical function and quality of life outcomes
Significant reductions in disability index (HAQ-DI) and fatigue (FACIT-Fatigue) scores were observed in patients treated with rituximab compared to patients treated with methotrexate alone. The proportions of rituximab treated patients showing a minimal clinically important difference (MCID) in HAQ-DI (defined as an individual total score decrease of >0.22) was also higher than among patients receiving methotrexate alone (Table 17).
Significant improvement in health related quality of life was also demonstrated with significant improvement in both the physical health score (PHS) and mental health score (MHS) of the SF-36. Further, significantly higher proportion of patients achieved MCIDs for these scores (Table 17).
Table 17. Physical function and quality of life outcomes at week 24 in Trial 1:
| Outcome† | Placebo+MTX | Rituximab+MTX (2 x 1000 mg) |
|---|---|---|
| Mean change in HAQ‐DI | n=201 0.1 | n=298 ‐0.4*** |
| % HAQ‐DI MCID | 20% | 51% |
| Mean change in FACIT‐T | ‐0.5 | -9.1*** |
| Mean change in SF‐36 PHS | n=197 0.9 | n=294 5.8*** |
| % SF‐36 PHS MCID | 13% | 48%*** |
| Mean change in SF‐36 MHS | 1.3 | 4.7** |
| % SF‐36 MHS MCID | 20% | 38%* |
† Outcome at 24 weeks
Significant difference from placebo at the primary time point: p<0.05, **p<0.001 **p≤0.0001
MCID HAQ-DI ≥0.22, MCID SF-36 PHS >5.42, MCID SF-36 MHS >6.33
Efficacy in autoantibody (RF and or anti-CCP) seropositive patients
Patients seropositive to Rheumatoid Factor (RF) and/or anti-Cyclic Citrullinated Peptide (anti-CCP) who were treated with rituximab in combination with methotrexate showed an enhanced response compared to patients negative to both.
Efficacy outcomes in rituximab treated patients were analysed based on autoantibody status prior to commencing treatment. At Week 24, patients who were seropositive to RF and/or anti-CCP at baseline had a significantly increased probability of achieving ACR20 and 50 responses compared to seronegative patients (p=0.0312 and p=0.0096) (Table 18). These findings were replicated at Week 48, where autoantibody seropositivity also significantly increased the probability of achieving ACR70. At week 48 seropositive patients were 2-3 times more likely to achieve ACR responses compared to seronegative patients. Seropositive patients also had a significantly greater decrease in DAS28-ESR compared to seronegative patients (Figure 1).
Table 18. Summary of efficacy by baseline autoantibody status:
Figure 1. Change from baseline of DAS28-ESR by baseline autoantibody status:
Long-term efficacy with multiple course therapy
Treatment with rituximab in combination with methotrexate over multiple courses resulted in sustained improvements in the clinical signs and symptoms of RA, as indicated by ACR, DAS28-ESR and EULAR responses which was evident in all patient populations studied (Figure 2). Sustained improvement in physical function as indicated by the HAQ-DI score and the proportion of patients achieving MCID for HAQ-DI were observed.
Figure 2. ACR responses for 4 treatment courses (24 weeks after each course (within patient, within visit) in patients with an inadequate response to TNF-inhibitors (n=146):
Clinical laboratory findings
A total of 392/3095 (12.7%) patients with rheumatoid arthritis tested positive for ADA in clinical trials following therapy with rituximab. The emergence of ADA was not associated with clinical deterioration or with an increased risk of reactions to subsequent infusions in the majority of patients. The presence of ADA may be associated with worsening of infusion or allergic reactions after the second infusion of subsequent courses.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with rituximab in all subsets of the paediatric population with autoimmune arthritis. See section 4.2 for information on paediatric use.
Clinical experience in granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA)
Adult induction of remission
In GPA/MPA Study 1,a total of 197 patients aged 15 years or older with severe active GPA (75%) and MPA (24%) were enrolled and treated in an active-comparator, randomised, double-blind, multicentre, non-inferiority trial.
Patients were randomised in a 1:1 ratio to receive either oral cyclophosphamide daily (2 mg/kg/day) for 3-6 months or rituximab (375 mg/m²) once weekly for 4 weeks. All patients in the cyclophosphamide arm received azathioprine maintenance therapy during follow-up. Patients in both arms received 1000 mg of pulse intravenous (IV) methylprednisolone (or another equivalent-dose glucocorticoid) per day for 1 to 3 days, followed by oral prednisone (1 mg/kg/day, not exceeding 80 mg/day). Prednisone tapering was to be completed by 6 months from the start of study treatment.
The primary outcome measure was achievement of complete remission at 6 months defined as a Birmingham Vasculitis Activity Score for Wegener's granulomatosis (BVAS/WG) of 0, and off glucocorticoid therapy. The pre-specified non-inferiority margin for the treatment difference was 20%. The trial demonstrated non-inferiority of rituximab to cyclophosphamide for complete remission (CR) at 6 months (Table 19).
Efficacy was observed both for patients with newly diagnosed disease and for patients with relapsing disease (Table 20).
Table 19. Percentage of adult patients who achieved complete remission at 6 months (Intent-to-treat population*):
| Rituximab (n=99) | Cyclophosphamide (n=98) | Treatment difference (Rituximab - cyclophosphamide) | |
|---|---|---|---|
| Rate | 63.6% | 53.1% | 10.6% 95.1%b CI (-3.2%, 24.3%)a |
CI = confidence interval.
* Worst case imputation
a Non-inferiority was demonstrated since the lower bound (-3.2%) was higher than the pre-determined non-inferiority margin (-20%).
b The 95.1% confidence level reflects an additional 0.001 alpha to account for an interim efficacy analysis.
Table 20. Complete remission at 6-months by disease status:
| Rituximab | Cyclophosphamide | Difference (CI 95%) | |
|---|---|---|---|
| All patients newly diagnosed relapsing | n=99 n=48 n=51 | n=98 n=48 n=50 | |
| Complete remission | |||
| All patients | 63.6% | 53.1% | 10.6% (-3.2, 24.3) |
| Newly diagnosed | 60.4% | 64.6% | -4.2% (-23.6, 15.3) |
| Relapsing | 66.7% | 42.0% | 24.7% (5.8, 43.6) |
Worst case imputation is applied for patients with missing data.
Complete remission at 12 and 18 months
In the rituximab group, 48% of patients achieved CR at 12 months, and 39% of patients achieved CR at 18 months. In patients treated with cyclophosphamide (followed by azathioprine for maintenance of complete remission), 39% of patients achieved CR at 12 months, and 33% of patients achieved CR at 18 months. From month 12 to month 18, 8 relapses were observed in the rituximab group compared with four in the cyclophosphamide group.
Laboratory evaluations
A total of 23/99 (23%) rituximab-treated patients from the induction of remission trial tested positive for ADA by 18 months. None of the 99 rituximab-treated patients were ADA positive at screening. There was no apparent trend or negative impact of the presence of ADA on safety or efficacy in the induction of the remission trial.
Adult maintenance treatment
A total of 117 patients (88 with GPA, 24 with MPA, and 5 with renal-limited ANCA-associated vasculitis) in disease remission were randomised to receive azathioprine (59 patients) or rituximab (58 patients) in a prospective, multi-center, controlled, open-label study. Included patients were 21 to 75 years of age and had newly diagnosed or relapsing disease in complete remission after combined treatment with glucocorticoids and pulse cyclophosphamide. The majority of patients were ANCA-positive at diagnosis or during the course of their disease; had histologically confirmed necrotizing small-vessel vasculitis with a clinical phenotype of GPA or MPA, or renal limited ANCA-associated vasculitis; or both.
Remission-induction therapy included intravenous prednisone, administered as per the investigator's discretion, preceded in some patients by methylprednisolone pulses, and pulse cyclophosphamide until remission was attained after 4 to 6 months. At that time, and within a maximum of 1 month after the last cyclophosphamide pulse, patients were randomly assigned to receive either rituximab (two 500 mg intravenous infusions separated by two weeks (on Day 1 and Day 15) followed by 500 mg intravenous every 6 months for 18 months) or azathioprine (administered orally at a dose of 2 mg/kg/day for 12 months, then 1.5 mg/kg/day for 6 months, and finally 1 mg/kg/day for 4 months (treatment discontinuation after these 22 months)). Prednisone treatment was tapered and then kept at a low dose (approximately 5 mg per day) for at least 18 months after randomization. Prednisone dose tapering and the decision to stop prednisone treatment after month 18 were left at the investigator's discretion.
All patients were followed until month 28 (10 or 6 months, respectively, after the last rituximab infusion or azathioprine dose). Pneumocystis jirovecii pneumonia prophylaxis was required for all patients with CD4+ T-lymphocyte counts less than 250 per cubic millimeter.
The primary outcome measure was the rate of major relapse at month 28.
Results
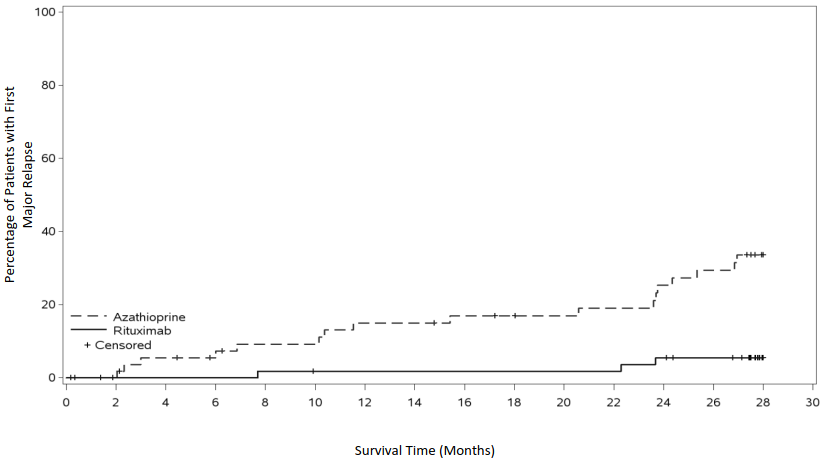
At month 28, major relapse (defined by the reappearance of clinical and/or laboratory signs of vasculitis activity ([BVAS] >0) that could lead to organ failure or damage or could be life threatening) occurred in 3 patients (5%) in the rituximab group and 17 patients (29%) in the azathioprine group (p=0.0007). Minor relapses (not life threatening and not involving major organ damage) occurred in seven patients in the rituximab group (12%) and eight patients in the azathioprine group (14%).
The cumulative incidence rate curves showed that time to first major relapse was longer in patients with rituximab starting from month 2 and was maintained up to month 28 (Figure 3).
Figure 3. Cumulative incidence over time of first major relapse:
| Number of Subjects with Major Relapse | |||||||||||||||
| Azathioprine | 0 | 0 | 3 | 3 | 5 | 5 | 8 | 8 | 9 | 9 | 9 | 10 | 13 | 15 | 17 |
| Rituximab | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 3 |
| Number of subjects at risk | |||||||||||||||
| Azathioprine | 59 | 56 | 52 | 50 | 47 | 47 | 44 | 44 | 42 | 41 | 40 | 39 | 36 | 34 | 0 |
| Rituximab | 58 | 56 | 56 | 56 | 55 | 54 | 54 | 54 | 54 | 54 | 54 | 54 | 52 | 50 | 0 |
Note: Patients were censored at month 28 if they had no event.
Laboratory evaluations
A total of 6/34 (18%) of rituximab treated patients from the maintenance therapy clinical trial developed ADA. There was no apparent trend or negative impact of the presence of ADA on safety or efficacy in the maintenance therapy clinical trial.
Paediatric population
Granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA)
Study WA25615 (PePRS) was a multicenter, open-label, single-arm, uncontrolled study in 25 paediatric patients (≥2 to <18 years old) with severe, active GPA or MPA. The median age of patients in the study was: 14 years (range: 6-17 years) and the majority of patients (20/25 [80%]) were female. A total of 19 patients (76%) had GPA and 6 patients (24%) had MPA at baseline. Eighteen patients (72%) had newly diagnosed disease upon study entry (13 patients with GPA and 5 patients with MPA) and 7 patients had relapsing disease (6 patients with GPA and 1 patient with MPA).
The study design consisted of an initial 6-month remission induction phase, with a minimum 18-month follow-up, up to a maximum of 54 months (4.5 years) overall. Patients were to receive a minimum of 3 doses of intravenous methylprednisolone (30 mg/kg/day, not exceeding 1 g/day) prior to the first rituximab intravenous infusion. If clinically indicated, additional daily doses (up to three), of intravenous methylprednisolone could be given. The remission induction regimen consisted of four once weekly intravenous infusions of rituximab at a dose of 375 mg/m² BSA, on study days 1, 8, 15 and 22 in combination with oral prednisolone or prednisone at 1 mg/kg/day (max 60 mg/day) tapered to 0.2 mg/kg/day minimum (max 10 mg/day) by Month 6. After the remission induction phase, patients could, at the discretion of the investigator, receive subsequent rituximab infusions on or after Month 6 to maintain PVAS remission and control disease activity (including progressive disease or flare) or to achieve first remission.
All 25 patients completed all four once weekly intravenous infusions for the 6-month remission induction phase. A total of 24 out of 25 patients completed at least 18 months of follow-up.
The objectives of this study were to evaluate safety, PK parameters, and efficacy of rituximab in paediatric GPA and MPA patients (≥2 to <18 years old). The efficacy objectives of the study were exploratory and principally assessed using the Pediatric Vasculitis Activity Score (PVAS) (Table 21).
Cumulative Glucocorticoid dose (intravenous and Oral) by Month 6
Twenty-four out of 25 patients (96%) in Study WA25615 achieved oral glucocorticoid taper to 0.2 mg/kg/day (or less than or equal to 10 mg/day, whichever was lower) at or by Month 6 during the protocol- defined oral steroid taper.
A decrease in median overall oral glucocorticoid use was observed from Week 1 (median = 45 mg prednisone equivalent dose [IQR: 35 – 60]) to Month 6 (median = 7.5 mg [IQR: 4-10]), which was subsequently maintained at Month 12 (median = 5 mg [IQR: 2-10]) and Mmonth 18 (median =5 mg [IQR: 1-5]).
Follow-Up Treatment
During the Overall Study Period, patients received between 4 and 28 infusions of rituximab (up to 4.5 yrs [53.8 months]). Patients received up to 375 mg/m² x 4 of rituximab, approximately every 6 months at the discretion of the investigator. In total, 17 out of 25 patients (68%) received additional rituximab treatment at or post Month 6 until the Common Close Out, 14 out of these 17 patients received additional rituximab treatment between Month 6 and Month 18.
Table 21. Study WA25615 (PePRS) - PVAS remission at Month 1, 2, 4, 6, 12 and 18:
| Study visit | Number of responders in PVAS remission* (response rate [%]) n=25 | 95% CIα |
|---|---|---|
| Month 1 | 0 | 0.0%, 13.7% |
| Month 2 | 1 (4.0%) | 0.1%, 20.4% |
| Month 4 | 5 (20.0%) | 6.8%, 40.7% |
| Month 6 | 13 (52.0%) | 31.3%, 72.2% |
| Month 12 | 18 (72.0%) | 50.6%, 87.9% |
| Month 18 | 18 (72.0%) | 50.6%, 87.9% |
* PVAS of 0 and achieved glucocorticoid taper to 0.2 mg/kg/day (or 10 mg/day, whichever is lower) at the assessment time-point.
α the efficacy results are exploratory and no formal statistical testing was performed for these endpoints rituximab, treatment (375 mg/m² x 4 infusions) up to Month 6 was identical for all patients. Follow-up treatment post Month 6
was at the discretion of the investigator.
Laboratory evaluations
A total of 4/25 patients (16%) developed ADA during the overall study period. Limited data shows there was no trend observed in the adverse reactions reported in ADA positive patients.
There was no apparent trend or negative impact of the presence of ADA on safety or efficacy in the paediatric GPA and MPA clinical trials.
The European Medicines Agency has waived the obligation to submit the results of studies with rituximab in paediatric population < 2 years of age in severe, active GPA or MPA. See section 4.2 for information on paediatric use.
Clinical experience in pemphigus vulgaris
PV Study 1 (Study ML22196)
The efficacy and safety of rituximab in combination with short-term, low-dose glucocorticoid (prednisone) therapy were evaluated in newly diagnosed patients with moderate to severe pemphigus (74 pemphigus vulgaris [PV] and 16 pemphigus foliaceus [PF]) in this randomised, open-label, controlled, multicenter study. Patients were between 19 and 79 years of age and had not received prior therapies for pemphigus. In the PV population, 5 (13%) patients in the rituximab group and 3 (8%) patients in the standard prednisone group had moderate disease and 33 (87%) patients in the rituximab group and 33 (92%) patients in the standard-dose prednisone group had severe disease according to disease severity defined by Harman's criteria.
Patients were stratified by baseline disease severity (moderate or severe) and randomised 1:1 to receive either rituximab and low-dose prednisone or standard-dose prednisone. Patients randomised to the rituximab group received an initial intravenous infusion of 1000 mg rituximab on Study Day 1 in combination with 0.5 mg/kg/day oral prednisone tapered off over 3 months if they had moderate disease or 1 mg/kg/day oral prednisone tapered off over 6 months if they had severe disease, and a second intravenous infusion of 1000 mg on Study Day 15. Maintenance infusions of rituximab 500 mg were administered at months 12 and 18. Patients randomised to the standard-dose prednisone group received an initial 1 mg/kg/day oral prednisone tapered off over 12 months if they had moderate disease or 1.5 mg/kg/day oral prednisone tapered off over 18 months if they had severe disease. Patients in the rituximab group who relapsed could receive an additional infusion of rituximab 1000 mg in combination with reintroduced or escalated prednisone dose. Maintenance and relapse infusions were administered no sooner than 16 weeks following the previous infusion.
The primary objective for the study was complete remission (complete epithelialisation and absence of new and/or established lesions) at month 24 without the use of prednisone therapy for two months or more (CRoff for ≥2 months).
PV Study 1 Results
The study showed statistically significant results of rituximab and low-dose prednisone over standard-dose prednisone in achieving CRoff ≥2 months at month 24 in PV patients (see Table 22).
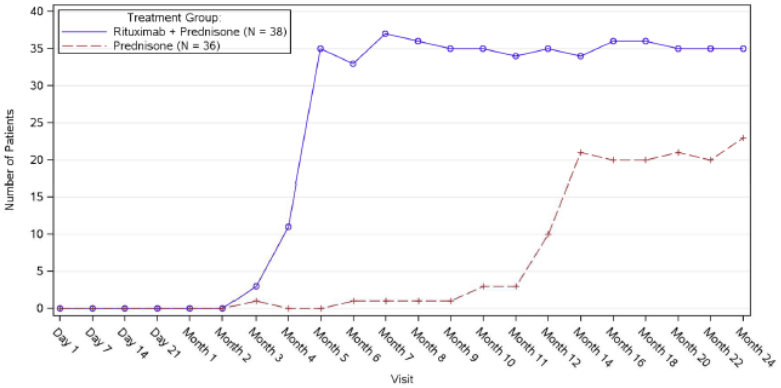
Table 22. Percentage of PV patients who achieved complete remission off corticosteroid therapy for two months or more at month 24 (Intent-to-Treat Population - PV):
| Rituximab + Prednisone N=38 | Prednisone N=36 | p-valuea | 95% CIb | |
|---|---|---|---|---|
| Number of responders (response rate [%]) | 34 (89.5%) | 10 (27.8%) | <0.0001 | 61.7% (38.4, 76.5) |
a p-value is from Fisher's exact test with mid-p correction
b 95% confidence interval is corrected Newcombe interval
The number of rituximab plus low-dose prednisone patients off prednisone therapy or on minimal therapy (prednisone dose of 10 mg or less per day) compared to standard-dose prednisone patients over the 24-month treatment period shows a steroid-sparing effect of rituximab (Figure 4).
Figure 4. Number of patients who were off or on minimal corticosteroid (≤10 mg/day) therapy over time:
Post-hoc retrospective laboratory evaluation
A total of 19/34 (56%) patients with PV, who were treated with rituximab, tested positive for ADA antibodies by 18 months. The clinical relevance of ADA formation in rituximab-treated PV patients is unclear.
PV Study 2 (Study WA29330)
In a randomized, double-blind, double-dummy, active-comparator, multicenter study, the efficacy and safety of rituximab compared with mycophenolate mofetil (MMF) were evaluated in patients with moderate-to-severe PV receiving 60-120 mg/day oral prednisone or equivalent (1.0-1.5 mg/kg/day) at study entry and tapered to reach a dose of 60 or 80 mg/day by Day 1. Patients had a confirmed diagnosis of PV within the previous 24 months and evidence of moderate-to-severe disease (defined as a total Pemphigus Disease Area Index, PDAI, activity score of ≥15).
One hundred and thirty-five patients were randomized to treatment with rituximab 1000 mg administered on Day 1, Day 15, Week 24 and Week 26 or oral MMF 2 g/day for 52 weeks in combination with 60 or 80 mg oral prednisone with the aim of tapering to 0 mg/day prednisone by Week 24.
The primary efficacy objective for this study was to evaluate at week 52, the efficacy of rituximab compared with MMF in achieving sustained complete remission defined as achieving healing of lesions with no new active lesions (i.e., PDAI activity score of 0) while on 0 mg/day prednisone or equivalent, and maintaining this response for at least 16 consecutive weeks, during the 52-week treatment period.
PV Study 2 Results
The study demonstrated the superiority of rituximab over MMF in combination with a tapering course of oral corticosteroids in achieving CR off corticosteroid ≥16 weeks at Week 52 in PV patients (Table 23).The majority of patients in the mITT population were newly diagnosed (74%) and 26% of patients had established disease (duration of illness ≥6 months and received prior treatment for PV).
Table 23. Percentage of PV Patients Who Achieved Sustained Complete Remission Off Corticosteroid Therapy for 16 Weeks or More at Week 52 (Modified Intent-to-Treat Population):
| Rituximab (N=62) | MMF (N=63) | Difference (95% CI) | p-value | |
|---|---|---|---|---|
| Number of responders (response rate [%]) | 25 (40.3%) | 6 (9.5%) | 30.80% (14.70%, 45.15%) | <0.0001 |
| Newly diagnosed patients | 19 (39.6%) | 4 (9.1%) | ||
| Patients with established disease | 6 (42.9%) | 2 (10.5%) |
MMF = Mycophenolate mofetil. CI = Confidence Interval.
Newly diagnosed patients = duration of illness <6 months or no prior treatment for PV.
Patients with established disease = duration of illness ≥6 months and received prior treatment for PV.
Cochran-Mantel-Haenszel test is used for p-value.
The analysis of all secondary parameters (including cumulative oral corticosteroid dose, the total number of disease flares, and change in health-related quality of life, as measured by the Dermatology Life Quality Index) verified the statistically significant results of rituximab compared to MMF. Testing of secondary endpoints were controlled for multiplicity.
Glucocorticoid exposure
The cumulative oral corticosteroid dose was significantly lower in patients treated with rituximab. The median (min, max) cumulative prednisone dose at Week 52 was 2775 mg (450, 22180) in the rituximab group compared to 4005 mg (900, 19920) in the MMF group (p=0.0005).
Disease flare
The total number of disease flares was significantly lower in patients treated with rituximab compared to MMF (6 vs. 44, p<0.0001) and there were fewer patients who had at least one disease flare (8.1% vs. 41.3%).
Laboratory evaluations
By week 52, a total of 20/63 (31.7%) (19 treatment-induced and 1 treatment-enhanced) rituximab -treated PV patients tested positive for ADA. There was no apparent negative impact of the presence of ADA on safety or efficacy in PV Study 2.
Pharmacokinetic properties
Adult Non-Hodgkin's lymphoma
Based on a population pharmacokinetic analysis in 298 NHL patients who received single or multiple infusions of rituximab as a single agent or in combination with CHOP therapy (applied rituximab doses ranged from 100 to 500 mg/m²), the typical population estimates of nonspecific clearance (CL1), specific clearance (CL2) likely contributed by B cells or tumour burden, and central compartment volume of distribution (V1) were 0.14 L/day, 0.59 L/day, and 2.7 L, respectively. The estimated median terminal elimination half-life of rituximab was 22 days (range, 6.1 to 52 days). Baseline CD19-positive cell counts and size of measurable tumour lesions contributed to some of the variability in CL2 of rituximab in data from 161 patients given 375 mg/m² as an intravenous infusion for 4 weekly doses. Patients with higher CD19-positive cell counts or tumour lesions had a higher CL2. However, a large component of inter-individual variability remained for CL2 after correction for CD19-positive cell counts and tumour lesion size. V1 varied by body surface area (BSA) and CHOP therapy. This variability in V1 (27.1% and 19.0%) contributed by the range in BSA (1.53 to 2.32 m²) and concurrent CHOP therapy, respectively, were relatively small. Age, gender and WHO performance status had no effect on the pharmacokinetics of rituximab. This analysis suggests that dose adjustment of rituximab with any of the tested covariates is not expected to result in a meaningful reduction in its pharmacokinetic variability.
Rituximab, administered as an intravenous infusion at a dose of 375 mg/m² at weekly intervals for 4 doses to 203 patients with NHL naive to rituximab, yielded a mean Cmax following the fourth infusion of 486 μg/mL (range, 77.5 to 996.6 μg/mL). Rituximab was detectable in the serum of patients 3–6 months after completion of last treatment.
Upon administration of rituximab at a dose of 375 mg/m² as an intravenous infusion at weekly intervals for 8 doses to 37 patients with NHL, the mean Cmax increased with each successive infusion, spanning from a mean of 243 μg/mL (range, 16–582 μg/mL) after the first infusion to 550 μg/mL (range, 171–1177 μg/mL) after the eighth infusion.
The pharmacokinetic profile of rituximab when administered as 6 infusions of 375 mg/m² in combination with 6 cycles of CHOP chemotherapy was similar to that seen with rituximab alone.
Paediatric DLBCL/BL/BAL/BLL
In the clinical trial studying paediatric DLBCL/BL/BAL/BLL, the PK was studied in a subset of 35 patients aged 3 years and older. The PK was comparable between the two age groups (≥3 to <12 years vs. ≥12 to <18 years). After two rituximab intravenous infusions of 375 mg/m² in each of the two induction cycles (cycle 1 and 2) followed by one rituximab intravenous infusion of 375 mg/m² in each of the consolidation cycles (cycle 3 and 4) the maximum concentration was highest after the fourth infusion (cycle 2) with a geometric mean of 347 μg/mL followed by lower geometric mean maximum concentrations thereafter (Cycle 4: 247 μg/mL). With this dose regimen, trough levels were sustained (geometric means: 41.8 μg/mL (pre-dose Cycle 2; after 1 cycle), 67.7 μg/mL (pre-dose Cycle 3, after 2 cycles) and 58.5 μg/mL (pre-dose Cycle 4, after 3 cycles)). The median elimination half-life in paediatric patients aged 3 years and older was 26 days.
The PK characteristics of rituximab in paediatric patients with DLBCL/BL/BAL/BLL were similar to what has been observed in adult NHL patients.
No PK data are available in the ≥6 months to <3 years age group, however, population PK prediction supports comparable systemic exposure (AUC, Ctrough) in this age group compared to ≥3 years (Table 24). Smaller baseline tumor size is related to higher exposure due to lower time dependent clearance, however, systemic exposures impacted by different tumor sizes remain in the range of exposure that was efficacious and had an acceptable safety profile.
Table 24. Predicted PK Parameters following the Rituximab Dosing Regimen in Paediatric DLBCL/BL/BAL/BLL:
| Age group | ≥6 mo to <3 years | ≥3 to <12 years | ≥12 to <18 years |
|---|---|---|---|
| Ctrough (μg/mL) | 47.5 (0.01-179) | 51.4 (0.00-182) | 44.1 (0.00-149) |
| AUC1-4cycles (μg*day/mL) | 13501 (278-31070) | 11609 (135-31157) | 11467 (110-27066) |
Results are presented as median (min – max); Ctrough is pre-dose Cycle 4.
Chronic lymphocytic leukaemia
Rituximab was administered as an intravenous infusion at a first-cycle dose of 375 mg/m² increased to 500 mg/m² each cycle for 5 doses in combination with fludarabine and cyclophosphamide in CLL patients. The mean Cmax (N=15) was 408 μg/mL (range, 97 – 764 μg/mL) after the fifth 500 mg/m² infusion and the mean terminal half-life was 32 days (range, 14 – 62 days).
Rheumatoid arthritis
Following two intravenous infusions of rituximab at a dose of 1000 mg, two weeks apart, the mean terminal half-life was 20.8 days (range, 8.58 to 35.9 days), mean systemic clearance was 0.23 L/day (range, 0.091 to 0.67 L/day), and mean steady-state distribution volume was 4.6 L (range, 1.7 to 7.51 L). Population pharmacokinetic analysis of the same data gave similar mean values for systemic clearance and half-life, 0.26 L/day and 20.4 days, respectively. Population pharmacokinetic analysis revealed that BSA and gender were the most significant covariates to explain inter-individual variability in pharmacokinetic parameters. After adjusting for BSA, male subjects had a larger volume of distribution and a faster clearance than female subjects. The gender-related pharmacokinetic differences are not considered to be clinically relevant and dose adjustment is not required. No pharmacokinetic data are available in patients with hepatic or renal impairment.
The pharmacokinetics of rituximab were assessed following two intravenous (IV) doses of 500 mg and 1000 mg on Days 1 and 15 in four studies. In all these studies, rituximab pharmacokinetics were dose proportional over the limited dose range studied. Mean Cmax for serum rituximab following first infusion ranged from 157 to 171 μg/mL for 2 × 500 mg dose and ranged from 298 to 341 μg/mL for 2 × 1000 mg dose. Following second infusion, mean Cmax ranged from 183 to 198 μg/mL for the 2 × 500 mg dose and ranged from 355 to 404 μg/mL for the 2 × 1000 mg dose. Mean terminal elimination half-life ranged from 15 to 16 days for the 2 × 500 mg dose group and 17 to 21 days for the 2 × 1000 mg dose group. Mean Cmax was 16 to 19% higher following second infusion compared to the first infusion for both doses.
The pharmacokinetics of rituximab were assessed following two intravenous doses of 500 mg and 1000 mg upon re-treatment in the second course. Mean Cmax for serum rituximab following first infusion was 170 to 175 μg/mL for 2 × 500 mg dose and 317 to 370 μg/mL for 2 × 1000 mg dose. Cmax following second infusion, was 207 μg/mL for the 2 × 500 mg dose and ranged from 377 to 386 μg/mL for the 2 × 1000 mg dose. Mean terminal elimination half-life after the second infusion, following the second course, was 19 days for 2 × 500 mg dose and ranged from 21 to 22 days for the 2 × 1000 mg dose. PK parameters for rituximab were comparable over the two treatment courses.
The pharmacokinetic (PK) parameters in the anti-TNF inadequate responder population, following the same dosage regimen (2 × 1000 mg, intravenous, 2 weeks apart), were similar with a mean maximum serum concentration of 369 μg/mL and a mean terminal half-life of 19.2 days.
Granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA)
Adult Population
Based on the population pharmacokinetic analysis of data in 97 patients with granulomatosis with 52 polyangiitis and microscopic polyangiitis who received 375 mg/m² rituximab once weekly for four doses, the estimated median terminal elimination half-life was 23 days (range, 9 to 49 days). Rituximab mean clearance and volume of distribution were 0.313 L/day (range, 0.116 to 0.726 L/day) and 4.50 L (range 2.25 to 7.39 L) respectively. Maximum concentration during the first 180 days (Cmax), minimum concentration at Day 180 (C180) and Cumulative area under the curve over 180 days (AUC180) were (median [range]) 372.6 (252.3-533.5) μg/mL, 2.1 (0-29.3) μg/mL and 10302 (3653-21874)μg/mL*days, respectively. The PK parameters of rituximab in adult GPA and MPA patients appear similar to what has been observed in rheumatoid arthritis patients.
Paediatric Population
Based on the population pharmacokinetic analysis of 25 children (6-17 years old) with GPA and MPA who received 375 mg/m² rituximab once weekly for four doses, the estimated median terminal elimination half-life was 22 days (range, 11 to 42 days). Rituximab mean clearance and volume of distribution were 0.221 L/day (range, 0. 0996 to 0.381 L/day) and 2.27 L (range 1.43 to 3.17 L) respectively. Maximum concentration during the first 180 days (Cmax), minimum concentration at Day 180 (C180) and Cumulative area under the curve over 180 days (AUC180) were (median [range]) 382.8 (270.6-513.6) μg/mL, 0.9 (0-17.7) μg/mL and 9787 (4838-20446) μg/mL*day, respectively.The PK parameters of rituximab in paediatric patients with GPA or MPA were similar to those in adults with GPA or MPA, once taking into account the BSA effect on clearance and volume of distribution parameters.
Pemphigus vulgaris
The PK parameters in adult PV patients receiving rituximab 1000 mg at Days 1, 15, 168, and 182 are summarized in Table 25.
Table 25. Population PK in adult PV patients from PV Study 2:
| Parameter | Infusion Cycle | |
|---|---|---|
| 1st cycle of 1000 mg Day 1 and Day 15 N=67 | 2nd cycle of 1000 mg Day 168 and Day 182 N=67 | |
| Terminal Half-life (days) Median (Range) | 21.0 (9.3-36.2) | 26.5 (16.4-42.8) |
| Clearance (L/day) Mean (Range) | 391 (159-1510) | 247 (128-454) |
| Central Volume of Distribution (L) Mean (Range) | 3.52 (2.48-5.22) | 3.52 (2.48-5.22) |
Following the first two rituximab administrations (at day 1 and 15, corresponding to cycle 1), the PK parameters of rituximab in patients with PV were similar to those in patients with GPA/MPA and patients with RA. Following the last two administrations (at day 168 and 182, corresponding to cycle 2), rituximab clearance decreased while the central volume of distribution remained unchanged.
Preclinical safety data
Rituximab has shown to be highly specific to the CD20 antigen on B cells. Toxicity studies in cynomolgus monkeys have shown no other effect than the expected pharmacological depletion of B cells in peripheral blood and in lymphoid tissue.
Developmental toxicity studies have been performed in cynomolgus monkeys at doses up to 100 mg/kg (treatment on gestation days 20-50) and have revealed no evidence of toxicity to the foetus due to rituximab. However, dose-dependent pharmacologic depletion of B cells in the lymphoid organs 53 of the foetuses was observed, which persisted post natally and was accompanied by a decrease in IgG level in the newborn animals affected. B cell counts returned to normal in these animals within 6 months of birth and did not compromise the reaction to immunisation.
Standard tests to investigate mutagenicity have not been carried out, since such tests are not relevant for this molecule. No long-term animal studies have been performed to establish the carcinogenic potential of rituximab.
Specific studies to determine the effects of rituximab on fertility have not been performed. In general toxicity studies in cynomolgus monkeys no deleterious effects on reproductive organs in males or females were observed.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.