TZIELD Solution for injection Ref.[107227] Active ingredients: Teplizumab
Source: FDA, National Drug Code (US) Revision Year: 2023
12. Clinical Pharmacology
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of teplizumab-mzwv or of other teplizumab products.
In the placebo-controlled study in patients aged 8 years of age and older with Stage 2 type 1 diabetes (Study TN-10) [see Clinical Studies (14)], approximately 57% of TZIELD-treated patients developed anti-teplizumab-mzwv antibodies, 46% of whom developed neutralizing antibodies. There is insufficient information to characterize the effects of ADA on pharmacokinetics, pharmacodynamics, or effectiveness of TZIELD. There was a higher incidence of rash in TZIELD-treated patients who developed anti-teplizumab-mzwv antibodies compared to those who did not develop anti-teplizumab-mzwv antibodies [see Adverse Reactions (6.1)].
12.1. Mechanism of Action
Teplizumab-mzwv binds to CD3 (a cell surface antigen present on T lymphocytes) and delays the onset of Stage 3 type 1 diabetes in adults and pediatric patients aged 8 years and older with Stage 2 type 1 diabetes. The mechanism may involve partial agonistic signaling and deactivation of pancreatic beta cell autoreactive T lymphocytes. Teplizumab-mzwv leads to an increase in the proportion of regulatory T cells and of exhausted CD8+ T cells in peripheral blood.
12.2. Pharmacodynamics
Clinical studies have shown that teplizumab-mzwv binds to CD3 molecules on the surface of both CD4+ and CD8+ T cells during treatment, with internalization of the teplizumab-mzwv/CD3 complex from the surface of T cells. Pharmacodynamic effects include lymphopenia in the absence of depletion of T cells with a nadir on the 5th day of dosing, during a 14-day course of TZIELD treatment [see Warnings and Precautions (5.3)]. Teplizumab-mzwv exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of teplizumab-mzwv have not been fully characterized.
12.3. Pharmacokinetics
Steady state concentrations of teplizumab-mzwv are not expected to be achieved during the 14-day course of TZIELD.
Distribution
The central volume of distribution (Vd) of teplizumab-mzwv was 2.27 L in a 60 kg subject.
Elimination
Teplizumab-mzwv showed saturable binding and elimination. The mean (SD) terminal elimination half-life and clearance of teplizumab-mzwv are 4.5 (0.2) days and 2.7 (0.8) L/day in a 60 kg subject, respectively.
Metabolism
Teplizumab-mzwv is expected to be metabolized into small peptides by catabolic pathways.
Specific Populations
No clinically significant differences in the pharmacokinetics of teplizumab-mzwv were observed based on age (8 to 35 years old), biologic sex, or racial groups (White, Asians).
BSA-based dosing normalizes the exposure to teplizumab-mzwv across body weight.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies have been performed to assess the carcinogenic potential of teplizumab-mzwv.
No studies have been performed to assess the mutagenic potential of teplizumab-mzwv. As an antibody, teplizumab-mzwv is not expected to interact directly with DNA.
Fertility and reproductive performance were unaffected in female and male mice that received a murine surrogate anti-mouse CD3 antibody administered by the subcutaneous route at doses up to 20 mg/kg.
14. Clinical Studies
The effectiveness of TZIELD was investigated in a randomized, double-blind, event-driven, placebo-controlled study (Study TN-10; NCT01030861) in 76 patients, 8 to 49 years of age with Stage 2 type 1 diabetes. Stage 2 type 1 diabetes was defined as having both of the following:
- Two or more of the following pancreatic islet autoantibodies:
- Glutamic acid decarboxylase 65 (GAD) autoantibodies
- Insulin autoantibody (IAA)
- Insulinoma-associated antigen 2 autoantibody (IA-2A)
- Zinc transporter 8 autoantibody (ZnT8A)
- Islet cell autoantibody (ICA)
- Dysglycemia on oral glucose tolerance testing
In this study, patients were randomized to receive TZIELD or placebo once daily by intravenous infusion for 14 days. Patients in the TZIELD group had a total drug exposure that was comparable to the total drug exposure achieved with the recommended total TZIELD dosage [see Dosage and Administration (2.4)]. The primary efficacy endpoint in this study was the time from randomization to development of Stage 3 type 1 diabetes diagnosis.
Baseline Patient Characteristics
In this study, 45% were female; 97% White, 1% Asian, and 1% reported multiracial background; 3% were Hispanic or Latino ethnicity; and 95% were from the United States. The median age was 14 years (72% were <18 years old) (Table 2).
Table 2. Baseline Age Characteristics of Adults and Pediatric Patients 8 Years of Age and Older with Stage 2 Type 1 Diabetes (Study TN-10)*:
| TZIELD N=44 | Placebo N=32 | |
|---|---|---|
| Age Group | ||
| ≥18 Years | 34% | 19% |
| <18 years | 66% | 81% |
| Pediatric Age Group Quartiles | ||
| 8 to <11 years | 21% | 25% |
| 11 to <14 years | 27% | 31% |
| 14 to <18 years | 18% | 25% |
* Intent to treat (ITT) population
Baseline Disease Characteristics
Table 3 displays the baseline disease characteristics in Study TN-10.
Table 3. Baseline Disease Characteristics of Adults and Pediatric Patients 8 Years of Age and Older with Stage 2 Type 1 Diabetes (Study TN-10)*:
| TZIELD N=44 | Placebo N=32 | |
|---|---|---|
| Glucose, mg/dL† | ||
| median (min, max) | 165 (115, 207) | 154 (103, 200) |
| HbA1c, % | ||
| median (min, max) | 5.2 (4.6, 6.1) | 5.3 (4.3, 5.6) |
| HLA-DR4 | ||
| Missing | 5% | 0 |
| Absent | 34% | 34% |
| Present | 61% | 66% |
| HLA-DR3 | ||
| Missing | 5% | 0 |
| Absent | 48% | 53% |
| Present | 48% | 47% |
| HLA-DR3/DR4 | ||
| Both DR3 and DR4 | 25% | 22% |
| DR3 only | 23% | 25% |
| DR4 only | 36% | 44% |
| Missing | 5% | 0 |
| Neither DR3 nor DR4 | 11% | 9% |
| Autoantibodies Positive (N) | ||
| 1 | 2% | 0 |
| 2 | 27% | 22% |
| 3 | 25% | 16% |
| 4 | 27% | 44% |
| 5 | 18% | 19% |
| Autoantibody Type Positive | ||
| GAD65 | 91% | 88% |
| IAA | 43% | 34% |
| IA-2A | 59% | 75% |
| ICA | 66% | 88% |
| ZnT8 | 73% | 75% |
Abbreviations: HbA1c=hemoglobin A1c, SD=standard deviation, HLA = human leukocyte antigen, GAD65=Glutamic acid decarboxylase 65 (GAD) autoantibodies, IAA=Insulin autoantibody, IA- 2A=Insulinoma-associated antigen 2 autoantibody, ZnT8A=Zinc transporter 8 autoantibody, ICA=Islet cell autoantibody
* Intent to treat (ITT) population
† The glucose data are area under the time-concentration curve (AUC) values from the oral glucose tolerance test
Efficacy Results
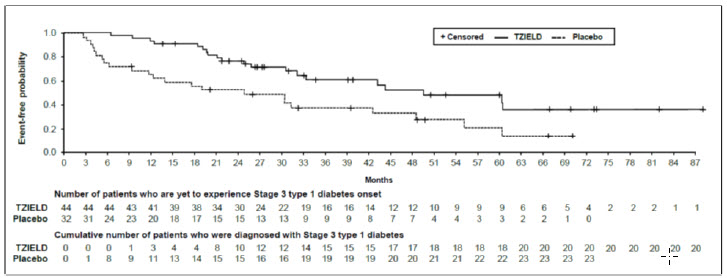
In Study TN-10, Stage 3 type 1 diabetes was diagnosed in 20 (45%) of the TZIELD-treated patients and in 23 (72%) of the placebo-treated patients. A Cox proportional hazards model, stratified by age and oral glucose tolerance test status at randomization, demonstrated that the median time from randomization to Stage 3 type 1 diabetes diagnosis was 50 months in the TZIELD group and 25 months in the placebo group, for a difference of 25 months. With a median follow-up time of 51 months, therapy with TZIELD resulted in a statistically significant delay in the development of Stage 3 type 1 diabetes, hazard ratio 0.41 (95% CI: 0.22 to 0.78; p=0.0066) (Figure 1).
Study TN-10 was not designed to assess whether there were differences in the effectiveness between subgroups based on demographic characteristics or baseline disease characteristics.
Figure 1. Kaplan-Meier Curve of Time to Diagnosis of Stage 3 Type 1 Diabetes in Adult and Pediatric Patients Aged 8 Years and Older with Stage 2 Type 1 Diabetes by Treatment Group (Study TN-10)1:
1 ITT population
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.