VELTASSA Powder for oral suspension Ref.[8679] Active ingredients: Patiromer
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Vifor Fresenius Medical Care Renal Pharma France, 100–101 Terrasse Boieldieu, Tour Franklin La Défense 8, 92042 Paris La Défense Cedex, France
Pharmacodynamic properties
Pharmacotherapeutic group: Drugs for treatment of hyperkalaemia and hyperphosphataemia
ATC code: V03AE09
Mechanism of action
Patiromer is a non-absorbed, cation exchange polymer that contains a calcium-sorbitol complex as a counterion.
Patiromer increases faecal potassium excretion through binding of potassium in the lumen of the gastrointestinal tract. Binding of potassium reduces the concentration of free potassium in the gastrointestinal lumen, resulting in a reduction of serum potassium levels.
Pharmacodynamic effects
In healthy adult subjects, patiromer caused a dose dependent increase in faecal potassium excretion, and a corresponding decrease in urinary potassium excretion with no change in serum potassium. 25.2 g of patiromer, administered once daily for 6 days, resulted in a mean increase in faecal potassium excretion of 1,283 mg/day, and a mean decrease in urinary potassium excretion of 1,438 mg/day. Daily urinary calcium excretion increased from baseline by 53 mg/day.
In an open label study to assess the time to onset of action, a statistically significant reduction in serum potassium in hyperkalaemic patients was observed at 7 hours after the first dose. Following discontinuation of patiromer, potassium levels remained stable for 24 hours after the last dose, then rose again during a 4-day observation period.
Clinical efficacy and safety
Overall, in the phase 2 and 3 clinical trials, 99.5% of patients were receiving RAAS inhibitor therapy at baseline, 87.0% had CKD with eGFR <60 mL/min/1.73 m² , 65.6% had diabetes mellitus and 47.5% had heart failure.
Study 1 (OPAL-HK)
The safety and efficacy of patiromer were demonstrated in a two-part, single blind randomised withdrawal study that evaluated this treatment in hyperkalaemic adult patients with chronic kidney disease (CKD) on stable doses of at least one RAAS inhibitor (i.e. angiotensin converting enzyme inhibitor [ACEI], angiotensin II receptor blocker [ARB] or mineralocorticoid receptor antagonist [MRA]).
In Part A, 243 patients were treated with patiromer for 4 weeks. Patients with a baseline serum potassium of 5.1 mEq/L to <5.5 mEq/L (mmol/L) received a starting dose of 8.4 g patiromer per day (as a divided dose) and patients with a baseline serum potassium of 5.5 mEq/L to <6.5 mEq/L received a starting dose of 16.8 g patiromer per day (as a divided dose). The dose was titrated, as needed, based on the serum potassium level, assessed starting on Day 3 and then at weekly visits to the end of the 4 week treatment period, with the aim of maintaining serum potassium in the target range (3.8 mEq/L to <5.1 mEq/L). The mean daily doses of patiromer were 13 g and 21 g in patients with serum potassium of 5.1 to <5.5 mEq/L and 5.5 to <6.5 mEq/L, respectively.
The mean age of patients was 64 years (54% aged 65 and over, 17% aged 75 and over), 58% of patients were men, and 98% were Caucasian. Approximately 97% of patients had hypertension, 57% had type 2 diabetes, and 42% had heart failure.
Mean serum potassium levels and change in serum potassium from Part A Baseline to Part A Week 4 is shown in Table 1. For the Part A secondary outcome, 76% (95% CI: 70%, 81%) of patients had a serum potassium in the target range of 3.8 mEq/L to <5.1 mEq/L at Part A Week 4.
Table 1. Patiromer treatment phase (Part A): primary endpoint:
| Baseline potassium | Overall population (n=237) | ||
| 5.1 to <5.5 mEq/L (n=90) | 5.5 to <6.5 mEq/L (n=147) | ||
| Serum potassium (mEq/L) | |||
| Baseline, mean (SD) | 5.31 (0.57) | 5.74 (0.40) | 5.58 (0.51) |
| Week 4 change from baseline, mean ± SE (95% CI) | −0.65 ± 0.05 (−0.74, −0.55) | −1.23 ± 0.04 (−1.31, −1.16) | −1.01 ± 0.03 (−1.07, −0.95) |
| p value | <0.001 | ||
In Part B, 107 patients with a Part A baseline serum potassium of 5.5 mEq/L to <6.5 mEq/L and whose serum potassium was in the target range (3.8 mEq/L to <5.1 mEq/L) at Part A Week 4 and still receiving RAAS inhibitor treatment were randomised to continue patiromer or to receive placebo for 8 weeks to evaluate the effect of withdrawing patiromer on serum potassium. In patients randomised to patiromer, the mean daily dose was 21 g at the start of Part B and during Part B.
The Part B primary endpoint was the change in serum potassium from Part B baseline to the earliest visit at which the patient’s serum potassium was first outside of the range of 3.8 to <5.5 mEq/L or to Part B Week 4 if the patient’s serum potassium remained in the range. In Part B, serum potassium in patients on placebo increased significantly relative to patients who remained on patiromer (p<0.001).
More placebo patients (91% [95% CI: 83%, 99%]) developed a serum potassium ≥5.1 mEq/L at any time during Part B than patiromer patients (43% [95% CI: 30%, 56%]), p<0.001. More placebo patients (60% [95% CI: 47%, 74%]) developed a serum potassium ≥5.5 mEq/L at any time during Part B than patiromer patients (15% [95% CI: 6%, 24%]), p<0.001.
The potential of patiromer to enable concomitant RAAS inhibitor treatment was also assessed in part B. Fifty two percent (52%) of subjects receiving placebo discontinued RAAS inhibitor treatment because of recurrent hyperkalaemia compared with 5% of subjects treated with patiromer.
Study 2 (AMETHYST-DN)
The effect of treatment with patiromer for up to 52 weeks was evaluated in an open label study of 304 hyperkalaemic patients with CKD and type 2 diabetes mellitus on stable doses of a RAAS inhibitor. The mean age of patients was 66 years (59.9% aged 65 and over, 19.7% aged 75 and over), 63% of patients were men, and all were Caucasian. Decreases in serum potassium with patiromer treatment were maintained over 1 year of chronic treatment as shown in Figure 1, with a low incidence of hypokalaemia (2.3%) and the majority of subjects reaching (97.7%) and maintaining target serum potassium levels (overall during maintenance period, serum potassium was within the target range for approximately 80% of the time). In patients with a baseline serum potassium of >5.0 to 5.5 mEq/L who received an initial dose of 8.4 g patiromer per day, the mean daily dose was 14 g; in those with a baseline serum potassium of >5.5 to <6.0 mEq/L who received an initial dose of 16.8 g patiromer per day, the mean daily dose was 20 g during the entire study.
Figure 1. Mean (95% CI) serum potassium over time:
Study 3 (PEARL-HF)
The ability of patiromer to enable concomitant spironolactone treatment was investigated in a randomised, double-blind, placebo-controlled study in heart failure patients who were clinically indicated to receive MRA. Patients initiated spironolactone at 25 mg/day at the same time as their randomised treatment (patiromer 12.6 g BID or placebo), and were up-titrated to 50 mg/day after Day 14 if serum potassium was >3.5 and ≤5.1 mEq/L. Of the 105 patients who were randomised and received study treatment (patiromer 56; placebo 49), mean age was 68.3 years, 60.6% were men, 97.1% were Caucasian, and mean eGFR was 81.3 mL/min. Mean baseline serum potassium values were 4.71 mEq/L for patiromer and 4.68 mEq/L for placebo.
The primary efficacy endpoint, change from baseline in serum potassium to the end of the 28-day treatment period, was significantly lower (p<0.001) in the patiromer group (LS mean [SEM]: −0.21 [0.07] mEq/L) as compared to the placebo group (LS mean [SEM]: +0.23 [0.07] mEq/L). There were also fewer patients in the patiromer group with serum potassium values >5.5 mEq/L (7.3% vs. 24.5%; p=0.027) and more patients on spironolactone 50 mg/day (90.9% versus 73.5%, p=0.022).
Study 4 (AMBER)
The ability of patiromer to enable concomitant spironolactone treatment in patients with resistant hypertension and CKD was further investigated in a randomised, double-blind, placebo-controlled study over 12 weeks. Normokalaemic patients initiated spironolactone at 25 mg per day together with their randomised treatment (patiromer 8.4 g or placebo per day). Patiromer/placebo was titrated weekly (up to 25.2 g per day) to maintain serum potassium ≥4.0 mEq/L and ≤5.1 mEq/L. At week 3 or after, spironolactone dose was increased to 50 mg per day for subjects with systolic blood pressure ≥120 mmHg and serum potassium ≤5.1 mEq/L.
Of the 295 randomized patients receiving study treatment (patiromer 147; placebo 148), mean age was 68.1 years, 51.9% were men, 98.3% were Caucasian, and mean eGFR was 35.73 mL/min/1.73 m² . At randomization, mean baseline serum potassium values were 4.74 mEq/L for patiromer and 4.69 mEq/L for placebo. The primary efficacy endpoint, the proportion of subjects remaining on spironolactone at Week 12, was significantly higher (p<0.0001) in the patiromer group (85.7%) compared to the placebo group (66.2%). Significantly more patients received spironolactone 50 mg/day (69.4% versus 51.4%).
Overall, patients in the patiromer group remained on spironolactone 7.1 days longer (95% CI 2.212.0; p=0.0045) compared to the placebo group and received significantly higher cumulative doses of spironolactone (2942.3 (SE 80.1) mg vs 2580.7 (SE 95.8) mg, p=0.0021).
There were also significantly fewer patients in the patiromer group with serum potassium values ≥5.5 mEq/L (35.4% vs. 64.2%, p<0.001).
At Week 12, the mean systolic blood pressure had decreased by 11.0 mmHg (SD 15.34) in the spironolactone + placebo group and by 11.3 mmHg (SD 14.11) in the spironolactone + patiromer group. These decreases from baseline were statistically significant within each treatment group (p<0.0001), but not statistically significant between the groups.
Effect of food
In an open-label study, 114 patients with hyperkalaemia were randomized to patiromer once daily with food or without food. Serum potassium at the end of treatment, the change from baseline in serum potassium, and the mean dose of patiromer were similar between groups.
Paediatric population
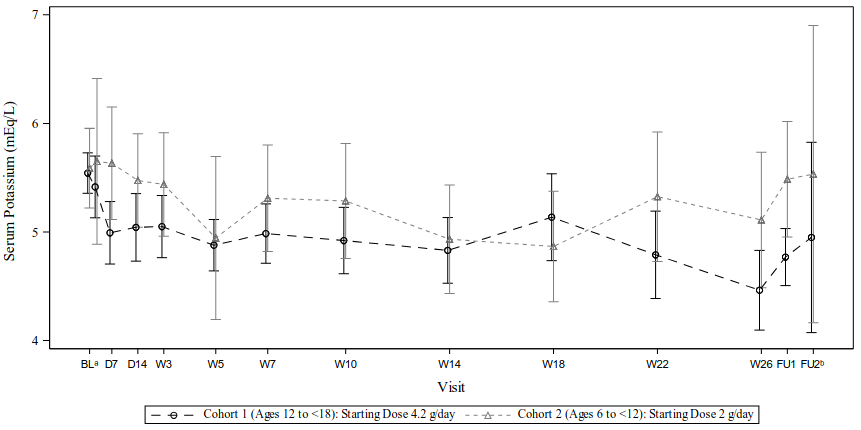
An open-label, multiple-dose study evaluated the efficacy, safety and tolerability of patiromer for oral suspension in children and adolescents 6 to <18 years of age with non-dialysis-dependent CKD and hyperkalaemia. Patients with severe gastrointestinal diagnosis or surgery were excluded. The study included 2 treatment phases; first, an initial 14-day dose finding phase, followed by an up to 24-week long-term (LT) treatment phase with a total of up to 26 treatment weeks. The study consisted of two age groups 12 to <18 years of age and 6 to <12 years of age, and the starting doses of patiromer in each age group were selected based on the median weights. Patiromer was given once daily as a powder for oral suspension.
Overall, 23 subjects (14 subjects aged 12 to <18 years and 9 subjects aged 6 to <12 years) completed the dose finding phase, and 21 subjects (12 subjects aged 12 to <18 years and 9 subjects aged 6 to <12 years) completed the LT treatment phase. No subject discontinued the study due to safety concerns.
The primary efficacy endpoint of this study was change from baseline in serum potassium levels at Day 14. In both age groups, a decrease in potassium levels was observed by day 14: mean (SD) potassium change from baseline was -0.50 (0.542) mEq/L in 12 to <18 years of age and -0.14 (0.553) mEq/L in 6 to <12 years of age) and was maintained throughout the study while on treatment (Figure 2).
The secondary efficacy endpoints were proportion of subjects with serum potassium within the target range (3.8 to 5.0 mEq/L) at Day 14 (dose finding phase) and by visit at any time through month 6 (LT treatment phase). In the group 12 to <18 years of age, 50.0% and 81.8% of subjects, achieved serum potassium levels within the target range at Day 14 and Week 26, respectively. The patiromer dose of 4.2 g/day appears to be an appropriate starting dose for this group. In the group 6 to <12 years of age, only 12.5% and 22.2% of patients, achieved serum potassium levels within the target range at Day 14 and Week 26, respectively.
Figure 2. Mean (±95% CI) serum potassium levels (safety population, N=23):
Notes:
a Baseline value was the last non-missing central laboratory value collected before the date and time of first dose of patiromer.
b Follow-up 2 was an optional site visit and could be a phone call.
Serum potassium data on or after the date of initiation of dialysis were excluded.
BL=Baseline; CI=Confidence interval; D=Day; FU=Follow-up; W=Week.
In the group 12 to <18 years of age, at Day 14 and at end of treatment, the median prescribed patiromer dose was 4.2 and 8.4 g/day, and the mean change from baseline in serum potassium was -0.50 and -1.08 mEq/L, respectively. In the group 6 to <12 years of age, at Day 14 and at end of treatment, the median prescribed patiromer dose was 6.0 and 8.0 g/day, and the mean change from baseline in potassium was -0.14 and -0.50 mEq/L, respectively. In subjects aged 12 to 17 years, the dose-response results qualitatively appeared to show, that a higher dose of patiromer was associated with a greater reduction in serum potassium in a treatment interval. However, in the group 6 to <12 years of age, the results of the dose finding were not conclusive. Further evaluation of patiromer in subjects aged 6 to <12 years is thus required to establish benefit risk.
The European Medicines Agency has deferred the obligation to submit the results of studies with Veltassa in children less than 6 years of age in the treatment of hyperkalaemia (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Patiromer works by binding potassium in the gastrointestinal tract and thus the serum concentration is not relevant for its efficacy. Due to the insolubility and nonabsorptive characteristics of this medicinal product, many classical pharmacokinetic studies cannot be carried out.
Patiromer is excreted approximately 24 to 48 hours after intake, based on average gastrointestinal transit time.
Preclinical safety data
In radiolabeled studies in rats and dogs, patiromer was not systemically absorbed and was excreted in the faeces. Quantitative whole-body autoradiography analysis in rats demonstrated that radioactivity was limited to the gastrointestinal tract, with no detectable level of radioactivity in any other tissues or organs.
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, toxicity to reproduction and development.
Patiromer was not genotoxic in the reverse mutation test (Ames assay), chromosome aberration or rat micronucleus assays.
Carcinogenicity studies have not been performed.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.