VERZENIOS Film-coated tablet Ref.[7607] Active ingredients: Abemaciclib
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Eli Lilly Nederland B.V., Papendorpseweg 83, 3528BJ Utrecht, The Netherlands
Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, protein kinases inhibitors
ATC code: L01EF03
Mechanism of action
Abemaciclib is a potent and selective inhibitor of cyclin-dependent kinases 4 and 6 (CDK4 and CDK6), and most active against Cyclin D1/CDK4 in enzymatic assays. Abemaciclib prevents retinoblastoma protein (Rb) phosphorylation, blocking cell cycle progression from the G1 to the S-phase of cell division, leading to suppression of tumour growth. In oestrogen receptor-positive breast cancer cell lines, sustained target inhibition with abemaciclib prevented rebound of Rb phosphorylation resulting in cell senescence and apoptosis. In vitro, Rb-negative and Rb-depleted cancer cell lines are generally less sensitive to abemaciclib. In breast cancer xenograft models, abemaciclib dosed daily without interruption at clinically relevant concentrations alone or in combination with anti-oestrogens resulted in reduction of tumour size.
Pharmacodynamic effects
In cancer patients, abemaciclib inhibits CDK4 and CDK6 as indicated by inhibition of phosphorylation of Rb and topoisomerase II alpha, which results in cell cycle inhibition upstream of the G1 restriction point.
Cardiac electrophysiology
The effect of abemaciclib on the QTcF interval was evaluated in 144 patients with advanced cancer. No large change (that is, >20 ms) in the QTcF interval was detected at the mean observed maximal steady state abemaciclib concentration following a therapeutic dosing schedule.
In an exposure-response analysis in healthy subjects at exposures comparable to a 200 mg twice-daily dose, abemaciclib did not prolong the QTcF interval to any clinically relevant extent.
Clinical efficacy and safety
Early breast cancer
Randomised Phase 3 Study monarchE: Verzenios in combination with endocrine therapy
The efficacy and safety of Verzenios in combination with adjuvant endocrine therapy was evaluated in monarchE, a randomised, open label, two cohort, phase 3 study, in women and men with HR-positive, HER2-negative, node positive early breast cancer at high risk of recurrence. High risk of recurrence in Cohort 1 was defined by clinical and pathological features: either ≥4 pALN (positive axillary lymph nodes), or 1-3 pALN and at least one of the following criteria: tumor size ≥ 5 cm or histological grade 3.
A total of 5 637 patients were randomised in a 1:1 ratio to receive 2 years of Verzenios 150 mg twice daily plus physician’s choice of standard endocrine therapy, or standard endocrine therapy alone. Randomization was stratified by prior chemotherapy, menopausal status, and region. Men were stratified as postmenopausal. Patients had completed definitive locoregional therapy (with or without neoadjuvant or adjuvant chemotherapy). Patients must have recovered from the acute side effects of any prior chemotherapy or radiotherapy. A washout period of 21 days after chemotherapy and 14 days after radiotherapy prior to randomization was required. Patients were allowed to receive up to 12 weeks of adjuvant endocrine therapy prior to randomisation. Adjuvant treatment with fulvestrant was not allowed as standard endocrine therapy. Patients with eastern cooperative oncology group (ECOG) Performance Status 0 or 1 were eligible. Patients with history of VTEs were excluded from the study. After the end of the study treatment period, in both treatment arms patients continued to receive adjuvant endocrine therapy for a cumulative duration of at least 5 years and up to 10 years, if medically appropriate. LHRH agonists were given when clinically indicated to pre- and perimenopausal women, and men.
Among the 5 637 randomised patients, 5 120 were enrolled in Cohort 1, representing 91% of the ITT population. In Cohort 1, patient demographics and baseline tumour characteristics were balanced between treatment arms. The median age of patients enrolled was approximately 51 years (range, 22-89 years), 15% of patients were 65 or older, 99% were women, 71% were Caucasian, 24% were Asian, and 5% Other. Forty three percent of patients were pre- or perimenopausal. Most patients received prior chemotherapy (36% neoadjuvant, 62% adjuvant), and prior radiotherapy (96%). Initial endocrine therapy received by patients included letrozole (39%), tamoxifen (31%), anastrozole (22%), or exemestane (8%).
Sixty-five percent of the patients had 4 or more positive lymph nodes, 41% had Grade 3 tumour, and 24% had pathological tumour size ≥5 cm at surgery.
The primary endpoint was invasive disease-free survival (IDFS) in ITT population defined as the time from randomization to the first occurrence of ipsilateral invasive breast tumour recurrence, regional invasive breast cancer recurrence, distant recurrence, contralateral invasive breast cancer, second 14 primary non-breast invasive cancer, or death attributable to any cause. Key secondary endpoint was distant relapse free survival (DRFS) in ITT population defined as time from randomization to the first occurrence of distant recurrence, or death attributable to any cause.
The primary objective of the study was met at the pre-planned interim analysis (16 Mar 2020 cut-off). A statistically significant improvement in IDFS was observed in patients who received Verzenios plus endocrine therapy versus endocrine therapy alone in the ITT population. The approval was granted for the large subpopulation, Cohort 1.
In a further analysis (01 April 2021 cut-off), 91% of the patients in Cohort 1 were off the 2-year study treatment period and the median duration of follow-up was 27.7 months.
Efficacy results in Cohort 1 are summarised in Table 9 and Figure 1.
Table 9. monarchE: Summary of efficacy data (Cohort 1 population):
| Verzenios plus endocrine therapy N=2 555 | Endocrine therapy alone N=2 565 | |
|---|---|---|
| Invasive disease-free survival (IDFS) | ||
| Number of patients with event (n, %) | 218 (8.5) | 318 (12.4) |
| Hazard ratio (95% CI) | 0.680 (0.572, 0.808) | |
| IDFS at 24 months ( %, 95% CI) | 92.6 (91.4, 93.5) | 89.6 (88.3, 90.8) |
| Distant relapse free survival (DRFS) | ||
| Number of patients with an event (n, %) | 179 (7.0) | 266 (10.4) |
| Hazard ratio (95% CI) | 0.669 (0.554, 0.809) | |
| DRFS at 24 months ( %, 95% CI) | 94.1 (93.0, 95.0) | 91.2 (90.0, 92.3) |
Abbreviation: CI = confidence interval.
Data cut-off date 01 Apr 2021
Figure 1. monarchE: Kaplan-Meier plot of IDFS (Investigator assessment, Cohort 1 population):
Abbreviations: CI = confidence interval; ET = endocrine therapy; HR = hazard ratio; IDFS = invasive disease-free survival; N = number of patients in the population.
Data cut-off date 01 April 2021
Benefit was observed across patient subgroups defined by geographic region, menopausal status and prior chemotherapy within Cohort 1.
Advanced or metastatic breast cancer
Randomised Phase 3 Study MONARCH 3: Verzenios in combination with aromatase inhibitors
The efficacy and safety of Verzenios in combination with an aromatase inhibitor (anastrozole or letrozole) was evaluated in MONARCH 3, a randomised, double-blind, placebo-controlled phase 3 study in women with HR positive, HER2 negative locally advanced or metastatic breast cancer who had not received prior systemic therapy in this disease setting. Patients were randomised in a 2:1 ratio to receive Verzenios 150 mg twice daily plus a non-steroidal aromatase inhibitor given daily at the recommended dose versus placebo plus a non-steroidal aromatase inhibitor according to the same schedule. The primary endpoint was investigator-assessed progression-free survival (PFS) evaluated according to RECIST 1.1; key secondary efficacy endpoints included objective response rate (ORR), clinical benefit rate (CBR) and overall survival (OS).
The median age of patients enrolled was 63 years (range 32-88). Approximately 39% of patients had received chemotherapy and 44% had received antihormonal therapy in the (neo)adjuvant setting. Patients with prior (neo)adjuvant endocrine therapy must have completed this therapy at least 12 months before study randomisation. The majority of patients (96%) had metastatic disease at baseline. Approximately 22% of patients had bone-only disease, and 53% patients had visceral metastases.
The study met its primary endpoint of improving PFS. Primary efficacy results are summarised in Table 10 and Figure 2.
Table 10. MONARCH 3: Summary of efficacy data (Investigator assessment, intent-to-treat population):
| Verzenios plus aromatase inhibitor | Placebo plus aromatase inhibitor | |
|---|---|---|
| Progression-free survival | N=328 | N=165 |
| Investigator assessment, number of events (%) | 138 (42.1) | 108 (65.5) |
| Median [months] (95% CI) | 28.18 (23.51, NR) | 14.76 (11.24, 19.20) |
| Hazard ratio (95% CI) and p-value | 0.540 (0.418, 0.698), p=0.000002 | |
| Independent radiographic review, number of events (%) | 91 (27.7) | 73 (44.2) |
| Median [months] (95% CI) | NR (NR, NR) | 19.36 (16.37, 27.91) |
| Hazard ratio (95% CI) and p-value | 0.465 (0.339, 0.636); p<0.000001 | |
| Objective response rateb [%] (95% CI) | 49.7 (44.3, 55.1) | 37.0 (29.6, 44.3) |
| Duration of response [months] (95% CI) | 27.39 (25.74, NR) | 17.46 (11.21, 22.19) |
| Objective response for patients with measurable diseasea | N=267 | N=132 |
| Objective response rateb [%] (95% CI) | 61.0 (55.2, 66.9) | 45.5 (37.0, 53.9) |
| Complete response, (%) | 3.4 | 0 |
| Partial response, (%) | 57.7 | 45.5 |
| Clinical benefit ratec (measurable disease) [%] (95% CI) | 79.0 (74.1, 83.9) | 69.7 (61.9, 77.5) |
a Measurable disease defined per RECIST version 1.1
b Complete response + partial response
c Complete response + partial response + stable disease for ≥6 months
N = number of patients; CI = confidence interval; NR = not reached.
Figure 2. MONARCH 3: Kaplan-Meier plot of progression-free survival (Investigator assessment, intent-to-treat population):
These results correspond to a clinically meaningful reduction in the risk of disease progression or death of 46% for patients treated with abemaciclib plus an aromatase inhibitor.
OS was not mature at the final PFS analysis (93 events observed across the two arms). The HR was 1.057 (95% CI: 0.683, 1.633), p=0.8017.
A series of prespecified subgroup PFS analyses showed consistent results across patient subgroups including age (<65 or ≥65 years), disease site, disease setting (de novo metastatic vs recurrent metastatic vs locally advanced recurrent), presence of measurable disease, progesterone receptor status, and baseline ECOG performance status. A reduction in the risk of disease progression or death was observed in patients with visceral disease, (HR of 0.567 [95% CI: 0.407, 0.789]), median PFS 21.6 months versus 14.0 months; in patients with bone-only disease (HR of 0.565 [95% CI: 0.306, 1.044]); and in patients with measurable disease (HR of 0.517 [95% CI: 0.392, 0.681]).
At the first OS interim analysis, 197 events were observed across the two arms and the HR was 0.786 (95% CI: 0.589, 1.049).
At the second OS interim analysis, 255 events were observed across the two arms and the HR was 0.754 (95% CI: 0.584, 0.974).
The results from the Final OS analysis were not statistically significant (summarised in Table 11 and Figure 3).
Table 11. MONARCH 3: Summary of Overall Survival data (Intent-to-Treat Population):
| Verzenios plus Anastrozole or Letrozole | Placebo plus Anastrozole or Letrozole | |
|---|---|---|
| Overall survival | N=328 | N=165 |
| Number of events (n, %) | 198 (60.4) | 116 (70.3) |
| Median OS [months] (95% CI) | 66.81 (59.21, 74.83) | 53.72 (44.75, 59.34) |
| Hazard ratio (95% CI) | 0.804 (0.637, 1.015) | |
Abbreviations: N = number of patients; CI = confidence interval; ITT = intent-to-treat; OS = overall survival.
Analyses for OS in patients with visceral disease showed an OS HR of 0.758 (95% CI: 0.558, 1.030). Median OS was 63.72 months in the abemaciclib plus AI arm and 48.82 months in the placebo plus AI arm. Similar to the ITT population, the results were not statistically significant.
Figure 3. MONARCH 3: Kaplan-Meier plot of overall survival (Intent-to-treat population):
Randomised Phase 3 Study MONARCH 2: Verzenios in combination with fulvestrant
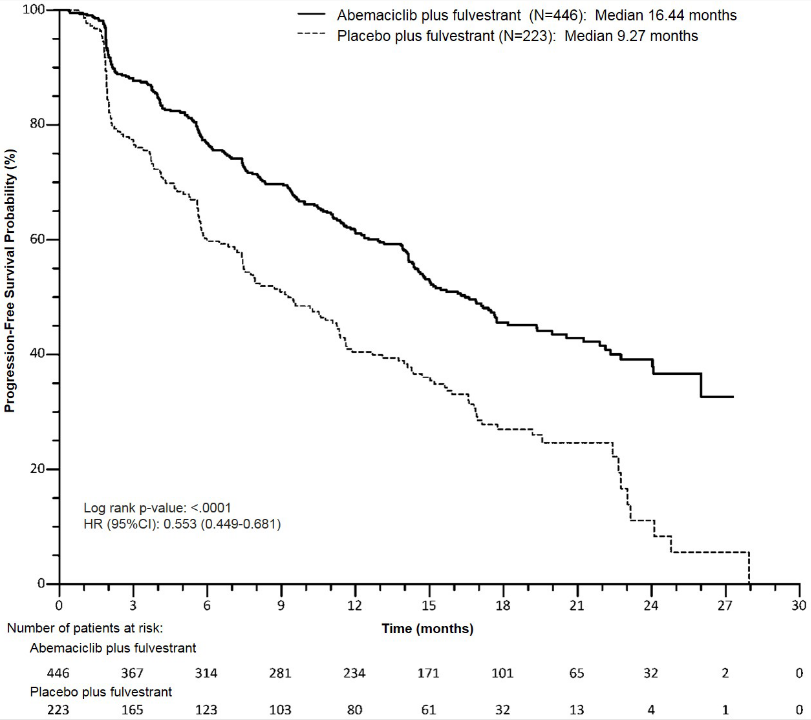
The efficacy and safety of Verzenios in combination with fulvestrant was evaluated in MONARCH 2, a randomised, double-blind, placebo-controlled phase 3 study in women with HR positive, HER2 negative locally advanced or metastatic breast cancer. Patients were randomised in a 2:1 ratio to receive Verzenios 150 mg twice daily plus fulvestrant 500 mg at intervals of one month, with an additional 500 mg dose given two weeks after the initial dose, versus placebo plus fulvestrant according to the same schedule. The primary endpoint was investigator-assessed PFS evaluated according to RECIST 1.1; key secondary efficacy endpoints included ORR, CBR and OS.
The median age of patients enrolled was 60 years (range, 32-91 years). In each treatment arm the majority of patients were white, and had not received chemotherapy for metastatic disease. 17% of patients were pre/perimenopausal on ovarian suppression with a GnRH agonist. Approximately 56% patients had visceral metastases. Approximately 25% of patients had primary endocrine resistance (progression on endocrine therapy within the first 2 years of adjuvant endocrine therapy or within the first 6 months of first line endocrine therapy for metastatic breast cancer) and for the majority, endocrine resistance developed later. 59% of patients had most recent endocrine therapy in the (neo)adjuvant setting, and 38% in metastatic setting.
The study met its primary endpoint of improving PFS. Primary efficacy results are summarised in Table 12 and Figure 4.
Table 12. MONARCH 2: Summary of efficacy data (Investigator assessment, intent-to-treat population):
| Verzenios plus fulvestrant | Placebo plus fulvestrant | |
|---|---|---|
| Progression-free survival | N=446 | N=223 |
| Investigator assessment, number of events (%) | 222 (49.8) | 157 (70.4) |
| Median [months] (95% CI) | 16.4 (14.4, 19.3) | 9.3 (7.4, 12.7) |
| Hazard ratio (95% CI) and p-value | 0.553 (0.449, 0.681), p=0.0000001 | |
| Independent radiographic review, number of events (%) | 164 (36.8) | 124 (55.6) |
| Median [months] (95% CI) | 22.4 (18.3, NR) | 10.2 (5.8, 14.0) |
| Hazard ratio (95% CI) and p-value | 0.460 (0.363, 0.584); p<0.000001 | |
| Objective response rateb [%] (95% CI) | 35.2 (30.8, 39.6) | 16.1 (11.3, 21.0) |
| Duration of response [months] (95% CI) | NR (18.05, NR) | 25.6 (11.9, 25.6) |
| Objective response for patients with measurable diseasea | N=318 | N=164 |
| Objective response rateb [%] (95% CI) | 48.1 (42.6, 53.6) | 21.3 (15.1, 27.6) |
| Complete response, (%) | 3.5 | 0 |
| Partial response, (%) | 44.7 | 21.3 |
| Clinical benefit ratec (measurable disease) [%] (95% CI) | 73.3 (68.4, 78.1) | 51.8 (44.2, 59.5) |
a Measurable disease defined per RECIST version 1.1
b Complete response + partial response
c Complete response + partial response + stable disease for ≥ 6 months
N = number of patients; CI = confidence interval; NR = not reached.
Figure 4. MONARCH 2: Kaplan-Meier plot of progression-free survival (Investigator assessment, intent-to-treat population):
These results correspond to a clinically meaningful reduction in the risk of disease progression or death of 44.7% for patients treated with Verzenios plus fulvestrant. Verzenios plus fulvestrant prolonged progression-free survival with neither a clinically meaningful, or significant detriment to health-related quality of life.
A series of prespecified subgroup PFS analyses showed consistent results across patient subgroups including age (<65 or ≥65 years), race, geographic region, disease site, endocrine therapy resistance, presence of measurable disease, progesterone receptor status, and menopausal status. A reduction in the risk of disease progression or death was observed in patients with visceral disease, (HR of 0.481 [95% CI: 0.369, 0.627]), median PFS 14.7 months versus 6.5 months; in patients with bone-only disease (HR of 0.543 [95% CI: 0.355, 0.833]); patients with measurable disease (HR of 0.523 [95% CI: 0.412, 0.644]). In patients who were pre/perimenopausal, the hazard ratio was 0.415 (95% CI: 0.246, 0.698); in patients who were progesterone receptor negative, the HR was 0.509 (95% CI: 0.325, 0.797).
In a sub-population with locally advanced or metastatic disease that had not received prior endocrine therapy, the PFS was also consistent.
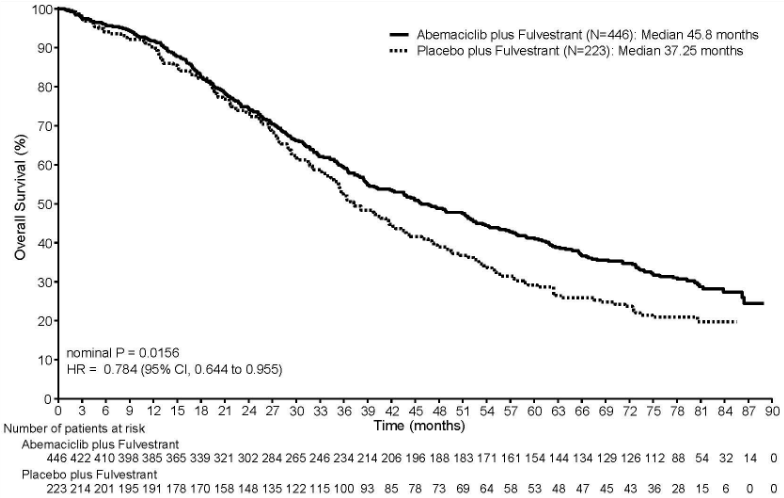
At the pre-specified interim OS analysis (20 June 2019 cut-off), the ITT population showed a statistically significant improvement in patients receiving Verzenios plus fulvestrant compared with those receiving placebo plus fulvestrant. The OS results are summarized in Table 13.
Table 13. MONARCH 2: Summary of overall survival data (Intent-to-treat population):
| Verzenios plus fulvestrant | Placebo plus fulvestrant | |
|---|---|---|
| Overall survival | N=446 | N=223 |
| Number of events (n, %) | 211 (47.3) | 127 (57.0) |
| Median OS [months] (95% CI) | 46.7 (39.2, 52.2) | 37.3 (34.4, 43.2) |
| Hazard ratio (95% CI) | 0.757 (0.606, 0.945) | |
| p-value | 0.0137 | |
N = number of patients; CI = confidence interval; OS = overall survival
Analyses for OS by stratification factors showed OS HR of 0.675 (95% CI: 0.511, 0.891) in patients with visceral disease, and 0.686 (95% CI: 0.451, 1.043) in patients with primary endocrine resistance.
At the pre-specified final OS analysis (18 March 2022 cut-off), 440 events were observed across the 2 arms. The improvement in OS previously observed at the interim OS analysis (20 June 2019 cut-off) was maintained in the abemaciclib plus fulvestrant arm compared to the placebo plus fulvestrant arm, with a HR of 0.784 (95% CI: 0.644, 0.955). Median OS was 45.8 months in the abemaciclib plus fulvestrant arm and 37.25 months in the placebo plus fulvestrant arm. The OS results are presented in Figure 5.
Figure 5. MONARCH 2: Kaplan-Meier plot of overall survival (Intent-to-treat population):
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Verzenios in all subsets of the paediatric population in breast cancer (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Absorption
Abemaciclib absorption is slow, with a Tmax of 8 hours and a mean absolute bioavailability of approximately 45%. In the therapeutic dose range of 50-200 mg, the increase in plasma exposure (AUC) and Cmax is approximately dose proportional. Steady state was achieved within 5 days following repeated twice daily dosing, and abemaciclib accumulated with a geometric mean accumulation ratio of 3.7 (58% CV) and 5.8 (65% CV) based on Cmax and AUC, respectively. A high-fat meal increased combined AUC of abemaciclib and its active metabolites by 9% and increased Cmax by 26%. These changes were not considered to be clinically relevant. Therefore, abemaciclib can be taken with or without food.
Distribution
Abemaciclib is highly bound to plasma proteins in humans (mean bound fraction approximately 96% to 98%). The geometric mean systemic volume of distribution is approximately 750 L (69% CV), indicating distribution of abemaciclib into tissues.
Concentrations of abemaciclib and its active metabolites in cerebrospinal fluid are comparable to unbound plasma concentrations.
Biotransformation
Hepatic metabolism is the main route of clearance for abemaciclib. Abemaciclib is metabolised to several metabolites primarily by cytochrome P450 (CYP) 3A4. The primary biotransformation is hydroxylation to a metabolite that circulates with an AUC that is 77% of parent drug. In addition, N-desethyl and N-desethylhydroxy metabolites circulate at AUCs that are 39% and 15% of parent drug. These circulating metabolites are active with similar potency to abemaciclib.
Elimination
The geometric mean hepatic clearance (CL) of abemaciclib was 21.8 L/h (39.8% CV), and the mean plasma elimination half-life for abemaciclib in patients was 24.8 hours (52.1% CV). After a single oral dose of [14C]-abemaciclib, approximately 81% of the dose was excreted in faeces and 3.4% excreted in urine. The majority of the dose eliminated in faeces was metabolites.
Special populations
Age, gender, and body weight
Age, gender, and body weight had no effect on the exposure of abemaciclib in a population pharmacokinetic analysis in patients with cancer (135 males and 859 females; age range 24-91 years; and body weight range 36-175 kg).
Hepatic impairment
Abemaciclib is metabolised in the liver. Mild (Child Pugh A) and moderate (Child Pugh B) hepatic impairment had no effect on the exposure of abemaciclib. In subjects with severe hepatic impairment (Child Pugh C), the AUC0-∞ of abemaciclib and potency adjusted unbound abemaciclib plus its active metabolites increased 2.1-fold and 2.4-fold, respectively. The half-life of abemaciclib increased from 24 to 55 hours (see section 4.2).
Renal impairment
Renal clearance of abemaciclib and its metabolites is minor. Mild and moderate renal impairment had no effect on the exposure of abemaciclib. There are no data in patients with severe renal impairment, end stage renal disease or in patients on dialysis.
Preclinical safety data
The primary target organ findings of potential relevance to humans occurred in the gastrointestinal tract, haematolymphopoietic organs, and male reproductive tract in mice, rats and dogs in studies up to 13 weeks duration. Effects in eyes and heart valves occurred only in rodents at clinically relevant exposure levels. Effects in lung and skeletal muscle occurred only in rodents at exposure levels at least 2-fold higher than human exposure levels. Effects in kidney occurred only in rodents at exposure levels at least 6-fold higher than human exposure levels. Complete or partial recovery was observed for all target organ findings at the end of the 28-day recovery period, with the exception of male reproductive tract effects.
Genotoxicity
Abemaciclib was not mutagenic in a bacterial reverse mutation (Ames) assay, was not clastogenic in an in vitro chromosomal aberration assay in human peripheral blood lymphocytes, and was not clastogenic in an in vivo rat bone marrow micronucleus assay.
Carcinogenicity
Abemaciclib was assessed for carcinogenicity in 2-year studies in rats and mice. In male rats, daily oral administration of abemaciclib resulted in benign testicular interstitial cell adenomas at exposures approximately 1.5 times human clinical exposure. In addition, interstitial cell hyperplasia was observed at exposures approximately 0.1 times human clinical exposure. It is unknown if these effects will translate to humans. There were no neoplastic findings in mice or in female rats that were due to administration of abemaciclib.
Impairment of fertility
Abemaciclib may impair fertility in males of reproductive potential. In repeat-dose toxicity studies up to 3 months duration, abemaciclib-related findings in the testis, epididymis, prostate, and seminal vesicle included decreased organ weights, intratubular cellular debris, hypospermia, tubular dilatation, atrophy, and degeneration/necrosis. These effects occurred in rats and dogs at exposures approximately 2 and 0.02 times human clinical exposure, respectively. In a rat male fertility study, abemaciclib had no effects on reproductive performance.
In a rat female fertility and early embryonic development study and in repeat-dose toxicity studies, abemaciclib did not have any effect on reproductive performance or any important effects on the female reproductive tract indicative of a risk of impaired fertility in females.
Developmental toxicity
Abemaciclib was teratogenic and caused decreased foetal weight at maternal exposures similar to the recommended human dose.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.