VIGAFYDE Oral solution Ref.[110641] Active ingredients: Vigabatrin
Revision Year: 2024
Product description
VIGAFYDE (vigabatrin) oral solution is an antiepileptic drug.
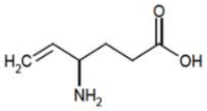
The chemical name of vigabatrin, is (±) 4-amino-5-hexenoic acid, a racemic mixture of R and S isomers. The molecular formula is C6H11NO2 and the molecular weight is 129.16.
It has the following structural formula:
Vigabatrin is a white to off-white powder which is freely soluble in water, slightly soluble in methyl alcohol, very slightly soluble in ethyl alcohol and chloroform, and insoluble in toluene and hexane. The pH of a 1% aqueous solution is about 6.9. The n-octanol/water partition coefficient of vigabatrin is about 0.011 (log P=-1.96) at physiologic pH. Vigabatrin melts with decomposition in a 3-degree range within the temperature interval of 171ºC to 176ºC. The dissociation constants (pKa) of vigabatrin are 4 and 9.7 at room temperature (25ºC).
Each mL of VIGAFYDE contains 100 mg of vigabatrin. The inactive ingredients are methylparaben; peppermint flavor; propylparaben; purified water; and sucralose.
| Dosage Forms and Strengths |
|---|
|
Oral Solution: 100 mg/mL clear, colorless to light yellow solution with a peppermint odor. |
| How Supplied |
|---|
|
VIGAFYDE (vigabatrin oral solution) contains 100 mg/mL vigabatrin. It is a clear, colorless to light yellow, peppermint-flavored solution supplied in a white opaque high-density polyethylene (HDPE) 150 mL bottle in a carton (NDC 80789-003-15). Manufactured for PYROS PHARMACEUTICALS, INC, Parsippany, NJ 07054, U.S.A. |
Drugs
| Drug | Countries | |
|---|---|---|
| VIGAFYDE | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.