VIZAMYL Solution for injection Ref.[10089] Active ingredients: Flutemetamol ¹⁸F
Source: FDA, National Drug Code (US) Revision Year: 2020
1. Indications and Usage
Vizamyl is indicated for Positron Emission Tomography (PET) imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer’s Disease (AD) and other causes of cognitive decline.
A negative Vizamyl scan indicates sparse to no neuritic plaques and is inconsistent with a neuropathological diagnosis of AD at the time of image acquisition; a negative scan result reduces the likelihood that a patient’s cognitive impairment is due to AD. A positive Vizamyl scan indicates moderate to frequent amyloid neuritic plaques; neuropathological examination has shown this amount of amyloid neuritic plaque is present in patients with AD, but may also be present in patients with other types of neurologic conditions as well as in older people with normal cognition. Vizamyl is an adjunct to other diagnostic evaluations.
Limitations of Use:
- A positive Vizamyl scan does not establish a diagnosis of AD or other cognitive disorder.
- Safety and effectiveness of Vizamyl have not been established for:
- Predicting development of dementia or other neurologic condition.
- Monitoring responses to therapies.
2. Dosage and Administration
2.1 Radiation Safety – Drug Handling
Vizamyl is a radioactive drug and should be handled with safety measures to minimize radiation exposure during administration [see Warnings and Precautions (5.3)]. Use waterproof gloves and effective shielding, including lead-glass syringe shields when handling and administering Vizamyl. To minimize radiation dose to the bladder, encourage patients to hydrate before and after Vizamyl administration in order to permit frequent voiding. Encourage patients to void before and after imaging with Vizamyl and frequently thereafter for 24 hours following Vizamyl administration.
Radiopharmaceuticals, including Vizamyl, should be used by or under the control of physicians who are qualified by specific training and experienced in the safe use and handling of radioactive materials, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radiopharmaceuticals.
2.2 Recommended Dosing and Administration Procedures
The recommended dose for Vizamyl is 185 megabecquerels (MBq) [5 millicuries (mCi)] in a maximum dose volume of 10 mL, administered as a single intravenous bolus within 40 seconds. The maximum mass dose is 20 micrograms. Follow the injection with an intravenous flush of 5 to 15 mL of 0.9% sterile sodium chloride injection.
- Use aseptic technique and radiation shielding to withdraw and administer Vizamyl solution.
- Calculate the necessary volume to administer based on calibration time and dose using a suitably calibrated instrument.
- Visually inspect Vizamyl for particulate matter and discoloration prior to administration. Do not administer Vizamyl if it contains particulate matter or is discolored [see Description (11)].
- Do not dilute Vizamyl.
- Dispose of unused product in a safe manner in compliance with applicable regulations [see How Supplied/Storage and Handling (16)].
2.3 Imaging Acquisition Guidelines
The recommended PET scan start time is 60 to 120 minutes after Vizamyl injection, using a PET scanner in 3-D mode with appropriate data corrections. A scan duration of 10 to 20 minutes is recommended. The time of initiation and the duration of the scan may vary depending on dose, imaging acquisition, and reconstruction parameters. Position the patient supine with the brain (including the cerebellum) within a single field of view. The patient’s head should be tilted so that the anterior commissure-posterior commissure (AC-PC) plane is at right angles to the bore-axis of the PET scanner, with the head positioned in a suitable head support. Reducing head movement with tape or other flexible head restraints may be employed. Iterative or filtered back-projection reconstruction is recommended with a slice thickness of 2 to 4 mm, matrix size of 128 × 128 with pixel sizes of approximately 2 mm. Where a post-smoothing filter is applied, a full width half maximum (FWHM) of not more than 5 mm is recommended; filter FWHM should be chosen to optimize the signal-to-noise ratio while preserving the sharpness of the reconstructed image.
2.4 Image Orientation and Display
Image Orientation
Orient axial and coronal images to show symmetry of brain structures, with equal heights of structures bilaterally. Orient sagittal images so that the head and neck are neither flexed nor extended; the anterior and posterior aspects of the corpus callosum should be parallel to the AC-PC line as shown in Figure 2.
Image Display
- Display images with all planes (axial, sagittal and coronal planes) linked by crosshairs.
- Select a color scale that provides a progression of low through high intensity (e.g., rainbow or Sokoloff). The selected color scale should (1) provide colors that allow the reader to discriminate intensity levels above and below the intensity level of the pons, (2) provide a color for regions with little or no amyloid binding such as the cerebellar cortex, and (3) provide a range of at least five distinct colors above 50 to 60% of the peak intensity.
- Display the reference scale. Adjust the color scale to set the pons to approximately 90% maximum intensity. The cerebellar cortex should represent approximately 20-30% of peak intensity on both negative and positive Vizamyl scans.
- Briefly display axial brain slices from bottom to top and look for signs of atrophy.
- Systematically review the following brain regions (recommended plane) for flutemetamol F 18 uptake as described in Image Interpretation below:
- Frontal lobes (axial, with optional sagittal plane view)
- Posterior cingulate and precuneus (sagittal, with optional coronal plane view)
- Lateral temporal lobes (axial, with optional coronal plane view)
- Inferolateral parietal lobes (coronal, with optional axial plane view)
- Striatum (axial, with optional sagittal plane view)
2.5 Image Interpretation
Vizamyl images should be interpreted only by readers who successfully complete the electronic or in-person training program provided by the manufacturer [see Warnings and Precautions (5.2)]. The objective of Vizamyl image interpretation is to provide an estimate of the brain β-amyloid neuritic plaque density, not to make a clinical diagnosis. Image interpretation is performed independently of a patient’s clinical features and relies upon recognition of image features in certain brain regions.
Image interpretation is based upon the distribution of radioactive signal within the brain; clinical information is not a component of image assessment [see Warnings and Precautions (5.2)]. Images are designated as positive or negative either by comparing radioactivity in cortical grey matter with activity in adjacent white matter, or based on the intensity in the five regions mentioned above. Signal uptake in the cerebellum does not contribute to scan interpretation (for example, a positive scan may show retained cerebellar grey-white contrast even when the cortical grey-white contrast is lost). Images should be viewed with the minimum image intensity set to zero and the maximum set such that the signal level in the easily identifiable pons is at 90% of maximum.
Negative scans show more radioactivity in white matter than in grey matter, creating clear grey-white matter contrast.
Specifically, a negative scan would have the following characteristics:
- frontal, lateral temporal, inferolateral parietal lobes: gradual gradient from bright intensity of the white matter to lower intensity at the periphery of the brain; distinct sulci with concave surfaces (white matter sulcal pattern),
and
- posterior cingulate and precuneus: grey matter uptake below 50-60% of peak intensity; gap of lower intensity separates two hemispheres on coronal view,
and
- striatum: approximately 50% of peak intensity or lower in the region between the higher intensities of the thalamus and frontal white matter (striatal “gap”)
Positive scans show at least one cortical region with reduction or loss of the normally distinct grey-white matter contrast. These scans have one or more regions with increased cortical grey matter signal (above 50-60% peak intensity) and/or reduced (or absent) grey-white matter contrast (white matter sulcal pattern is less distinct). A positive scan may have one or more regions in which grey matter radioactivity is as intense or exceeds the intensity in adjacent white matter.
Specifically, a positive scan would have the following characteristics:
- frontal, lateral temporal, or inferolateral parietal lobes: high intensity seen to the periphery of the brain, with sharp reduction of intensity at the brain margin; sulci not distinct due to fill-in by high intensity grey matter resulting in a convex surface at the edge of the brain,
or
- posterior cingulate and precuneus: grey matter uptake above 50-60% of peak intensity; high grey matter intensity that closes the gap between the two hemispheres on coronal view,
or
- striatum: intensity above 50-60% of peak intensity; gap between thalamus and frontal white matter not distinct
If any one of the brain regions systematically reviewed for flutemetamol F 18 uptake (see Image Orientation and Display above) is positive in either hemisphere, then the scan is considered positive. Otherwise, the scan is considered negative.
Among patients with clinically important β-amyloid neuritic plaques in the brain, the temporal lobes, parietal lobes, and striatum may not be as affected compared to other brain regions. Therefore, in some images, flutemetamol F 18 signal in these regions may not be as intense as in the frontal lobes or the posterior cingulate and precuneus regions.
Atrophy may affect the interpretability of scans, particularly in the frontal, temporal and parietal lobes [see Warnings and Precautions (5.2)]. For cases in which atrophy is apparent or suspected and there is uncertainty as to the location of the grey matter on the PET scan, examine the striatum for flutemetamol F 18 signal as it is less affected by atrophy than other regions of the brain.
If the patient’s MRI or CT brain images are available the interpreter should examine the CT or MRI images to clarify the relationship between PET flutemetamol F 18 uptake and grey matter anatomy.
Other factors that may affect the ability to interpret Vizamyl images include patient factors such as brain pathology, surgical changes, post-radiation therapy changes, and implants. Some scans may be difficult to interpret due to image noise, suboptimal patient positioning, or over-smoothing of the reconstructed image.
Figure 1. Axial view of negative (left) and positive (right) Vizamyl scans. The axial slices which cut through the frontal pole and inferior aspect of the splenium are shown using a rainbow color scale. The left image shows a white matter sulcal pattern at the frontal (f) and lateral temporal (lt) regions with a color intensity that tapers to the periphery, as well as less radioactivity in the striatal region(s). The right image shows absence of the white matter sulcal pattern with intensity radiating to a sharply defined convex edge, as well as more radioactivity in the striatum. In both the frontal and lateral temporal regions, the intensity is higher in the grey matter regions of the right image compared to those of the left image.
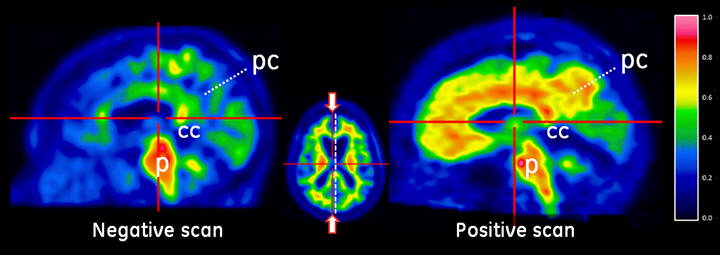
Figure 2. Sagittal view of negative (left) and positive (right) Vizamyl scans. The sagittal slices are slightly off midline in one hemisphere and shown using a rainbow color scale. In the posterior cingulate (pc) region, which is superior and posterior to the corpus callosum (cc), the left image shows intensity below 50% of peak intensity whereas the right image shows intensity above 60% of peak intensity. The pons (p) is set to approximately 90% of the maximum intensity.
Figure 3. Coronal view of negative (left) and positive (right) Vizamyl scans. The coronal slices are located posterior to the corpus callosum. The left image shows a white matter sulcal pattern in the inferior parietal (ip) regions that is not evident in the right image. Relative to the left image, the right image shows increased intensity in the posterior cinguli (pc) and increased radial extent of high intensity to the lateral surfaces of the parietal lobes particularly evident in the inferior parietal regions.
2.6 Radiation Dosimetry
The estimated absorbed radiation doses for adult patients following intravenous injection of Vizamyl are shown in Table 1. Values were calculated from human biodistribution data using OLINDA/EXM software and assuming emptying of the urinary bladder at 3.5 hour intervals.
The adult effective dose resulting from a 185-MBq (5-mCi) Vizamyl administration is 5.92 mSv. The use of a CT scan to calculate attenuation correction for reconstruction of Vizamyl images (as done in PET/CT imaging) will add radiation exposure at the level of approximately 0.1 mSv effective dose. Diagnostic head CT scans using helical scanners administer an average of 2.2 ± 1.3 mSv effective dose. The actual radiation dose is operator and scanner dependent.
Table 1. Adult Estimated Radiation Absorbed Vizamyl Doses in Organs/Tissues:
| Organ/Tissue | Absorbed Radiation Dose Per Unit Administered Activity microGy/MBq |
|---|---|
| Adrenals | 13 |
| Brain | 11 |
| Breasts | 5 |
| Gallbladder wall | 287 |
| Heart wall | 14 |
| Kidneys | 31 |
| Liver | 57 |
| Lower large intestine wall | 42 |
| Lungs | 16 |
| Muscle | 9 |
| Osteogenic cells | 11 |
| Ovaries | 25 |
| Pancreas | 15 |
| Red marrow | 13 |
| Skin | 5 |
| Small intestine wall | 102 |
| Spleen | 15 |
| Stomach wall | 12 |
| Testes | 8 |
| Thymus | 6 |
| Thyroid | 6 |
| Upper large intestine wall | 117 |
| Urinary bladder wall | 145 |
| Uterus | 25 |
| Total body | 12 |
| Effective Dose | 32 (microSv/MBq) |
10. Overdosage
The clinical consequence of overdose with Vizamyl has not been reported. It is unknown whether or not flutemetamol is dialyzable. The major risks of overdose relate predominantly to increased radiation exposure, with long-term risks for neoplasia. In case of overdose of radioactivity, hydration and frequent urination should be encouraged to minimize radiation exposure to the patient; care should be taken to avoid contamination from the radioactivity eliminated by the patient.
16.2. Storage and Handling
Storage
Store Vizamyl at 2° to 30°C (36° to 86°F). The product does not contain a preservative. Store Vizamyl within radiation shielding. Do not use Vizamyl after the expiry date and time stated on the label.
Handling
Vizamyl must not be diluted. This preparation is for use by persons licensed by the Nuclear Regulatory Commission or the relevant regulatory authority of an Agreement State.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.