VOSEVI Film-coated tablet Ref.[108058] Active ingredients: Sofosbuvir Sofosbuvir, Velpatasvir and Voxilaprevir Velpatasvir Voxilaprevir
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Gilead Sciences Ireland UC, Carrigtohill, County Cork, T45 DP77, Ireland
4.3. Contraindications
Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
Concomitant use with medicinal products that are strong P-glycoprotein (P-gp) and/or strong cytochrome P450 (CYP) inducers (e.g. carbamazepine, phenobarbital, phenytoin, rifampicin, rifabutin and St. John’s wort) (see section 4.5).
Concomitant use with rosuvastatin or dabigatran etexilate (see section 4.5).
Concomitant use with ethinylestradiol-containing medicinal products such as combined oral contraceptives or contraceptive vaginal rings or transdermal patches (see section 4.5).
4.4. Special warnings and precautions for use
Severe bradycardia and heart block
Life-threatening cases of severe bradycardia and heart block have been observed when sofosbuvir containing regimens are used in combination with amiodarone. Bradycardia has generally occurred within hours to days, but cases with a longer time to onset have been observed mostly up to 2 weeks after initiating HCV treatment.
Amiodarone should only be used in patients on Vosevi when other alternative anti-arrhythmic treatments are not tolerated or are contraindicated.
Should concomitant use of amiodarone be considered necessary, it is recommended that patients undergo cardiac monitoring in an in-patient setting for the first 48 hours of coadministration, after which outpatient or self-monitoring of the heart rate should occur on a daily basis through at least the first 2 weeks of treatment.
Due to the long half-life of amiodarone, cardiac monitoring as outlined above should also be carried out for patients who have discontinued amiodarone within the past few months and are to be initiated on Vosevi.
All patients with concurrent or recent use of amiodarone should be warned of the symptoms of bradycardia and heart block and should be advised to seek medical advice urgently should they experience them.
HCV/HBV co-infection
There are no data on the use of Vosevi in patients with HCV/hepatitis B virus (HBV) co-infection. Cases of HBV reactivation, some of them fatal, have been reported during or after treatment with DAAs. HBV screening should be performed in all patients before initiation of treatment. HCV/HBV co-infected patients are at risk of HBV reactivation, and should therefore be monitored and managed according to current clinical guidelines.
Renal impairment
Safety data are limited in patients with severe renal impairment (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m²) and ESRD requiring haemodialysis. Vosevi can be used in these patients with no dose adjustment when no other relevant treatment options are available (see sections 4.8, 5.1 and 5.2).
Hepatic impairment
No dose adjustment of Vosevi is required for patients with mild hepatic impairment (CPT Class A). Vosevi is not recommended in patients with moderate or severe hepatic impairment (CPT Class B or C) (see section 5.2).
Liver transplant patients
The safety and efficacy of Vosevi in the treatment of HCV infection in patients who are post-liver transplant have not been assessed. Treatment with Vosevi, in accordance with the recommended posology (see section 4.2), should be guided by an assessment of the potential benefits and risks for the individual patient.
Use with moderate P-gp inducers or moderate CYP inducers
Medicinal products that are moderate P-gp and/or moderate CYP inducers (e.g. efavirenz, modafinil, oxcarbazepine or rifapentine) may decrease sofosbuvir, velpatasvir and/or voxilaprevir plasma concentrations leading to reduced therapeutic effect of Vosevi. Co-administration of such medicinal products with Vosevi is not recommended (see section 4.5).
Use with strong OATP1B inhibitors
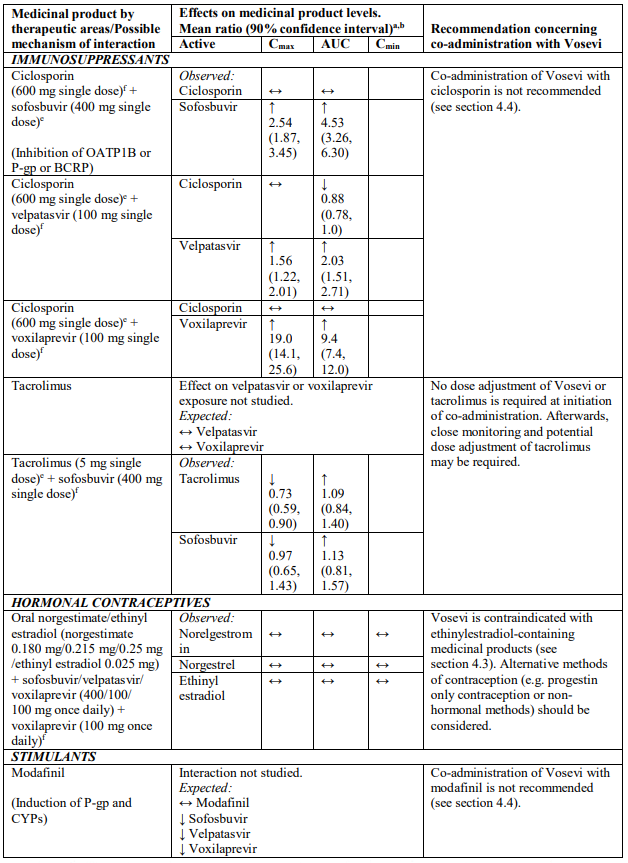
Medicinal products that are strong OATP1B inhibitors (e.g. ciclosporin) may substantially increase voxilaprevir plasma concentrations, the safety of which has not been established. Co-administration of strong OATP1B inhibitors with Vosevi is not recommended (see section 4.5).
Use with certain HIV antiretroviral regimens
Vosevi has been shown to increase tenofovir exposure when used together with an HIV regimen containing tenofovir disoproxil fumarate and a pharmacokinetic enhancer (ritonavir or cobicistat). The safety of tenofovir disoproxil fumarate in the setting of Vosevi and a pharmacokinetic enhancer has not been established. The potential risks and benefits associated with co-administration of Vosevi with the fixed-dose combination tablet containing elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate or tenofovir disoproxil fumarate given in conjunction with a boosted HIV protease inhibitor (e.g. darunavir) should be considered, particularly in patients at increased risk of renal dysfunction. Patients receiving Vosevi concomitantly with elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate or with tenofovir disoproxil fumarate and a boosted HIV protease inhibitor should be monitored for tenofovir-associated adverse reactions. Refer to tenofovir disoproxil fumarate, emtricitabine/tenofovir disoproxil fumarate, or elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate Summary of Product Characteristics for recommendations on renal monitoring.
Use in diabetic patients
Diabetics may experience improved glucose control, potentially resulting in symptomatic hypoglycaemia, after initiating HCV DAA treatment. Glucose levels of diabetic patients initiating DAA therapy should be closely monitored, particularly within the first 3 months, and their diabetic treatment modified when necessary. The physician in charge of the diabetic care of the patient should be informed when DAA therapy is initiated.
Excipients
Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucosegalactose malabsorption should not take this medicinal product.
This medicinal product contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’.
4.5. Interaction with other medicinal products and other forms of interaction
As Vosevi contains sofosbuvir, velpatasvir and voxilaprevir, any interactions that have been identified with these active substances individually may occur with Vosevi.
Pharmacokinetic interactions
Potential for Vosevi to affect other medicinal products
Velpatasvir and voxilaprevir are inhibitors of drug transporters P-gp, breast cancer resistance protein (BCRP), organic anion-transporting polypeptide (OATP) 1B1 and OATP1B3. Co-administration of Vosevi with medicinal products that are substrates of these transporters may increase the exposure of such medicinal products. Medicinal products that are sensitive substrates of these transporters and for which elevated plasma levels are associated with serious events are contraindicated (see Table 2). Dabigatran etexilate (P-gp substrate) and rosuvastatin (OATP1B and BCRP substrate) are contraindicated (see section 4.3 and Table 2).
Potential for other medicinal products to affect Vosevi
Sofosbuvir, velpatasvir and voxilaprevir are substrates of drug transporters P-gp and BCRP. Velpatasvir and voxilaprevir are substrates of drug transporters OATP1B1 and OATP1B3. In vitro, slow metabolic turnover of velpatasvir primarily by CYP2B6, CYP2C8 and CYP3A4 and of voxilaprevir primarily by CYP3A4 was observed.
Medicinal products that may decrease plasma exposure of Vosevi
Medicinal products that are strong inducers of P-gp and/or strong inducers of CYP2B6, CYP2C8, or CYP3A4 (e.g. carbamazepine, phenobarbital, phenytoin, rifampicin, rifabutin and St. John’s wort) may decrease plasma concentrations of sofosbuvir, velpatasvir and/or voxilaprevir leading to reduced therapeutic effect of Vosevi. The use of such medicinal products with Vosevi is contraindicated (see section 4.3 and Table 2).
Medicinal products that are moderate P-gp inducers and/or moderate CYP inducers (e.g. efavirenz, modafinil, oxcarbazepine or rifapentine) may decrease sofosbuvir, velpatasvir and/or voxilaprevir plasma concentrations leading to reduced therapeutic effect of Vosevi. Co-administration with such medicinal products is not recommended with Vosevi (see section 4.4 and Table 2).
Medicinal products that may increase plasma exposure of Vosevi
Co-administration with medicinal products that inhibit P-gp or BCRP may increase sofosbuvir, velpatasvir or voxilaprevir plasma concentrations. Medicinal products that inhibit OATP1B, CYP2B6, CYP2C8, or CYP3A4 may increase plasma concentrations of velpatasvir or voxilaprevir. The use of strong inhibitors of OATP1B (e.g. ciclosporin) with Vosevi is not recommended (see section 4.4 and Table 2). Clinically significant medicinal product interactions with Vosevi mediated by P-gp, BCRP and CYP inhibitors are not expected. Vosevi may be co-administered with P-gp, BCRP and CYP inhibitors.
Pharmacodynamic interactions
Patients treated with vitamin K antagonists
As liver function may change during treatment with Vosevi, close monitoring of International Normalised Ratio (INR) values is recommended.
Impact of DAA therapy on medicinal products metabolized by the liver
The pharmacokinetics of medicinal products that are metabolized by the liver (e.g. immunosuppressive agents such as calcineurin inhibitors) may be impacted by changes in liver function during DAA therapy, related to clearance of HCV.
Patients treated with ethinylestradiol-containing medicinal products
Concomitant use with ethinylestradiol-containing medicinal products may increase the risk of alanine aminotransferase (ALT) elevations and is contraindicated (see section 4.3 and Table 2).
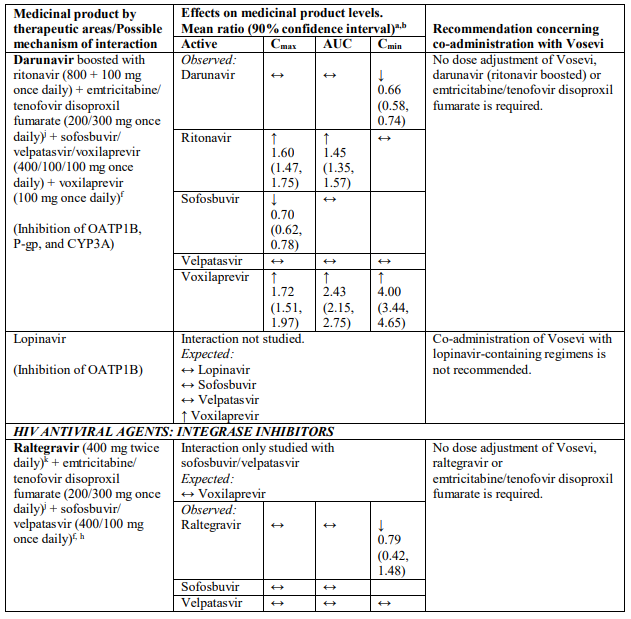
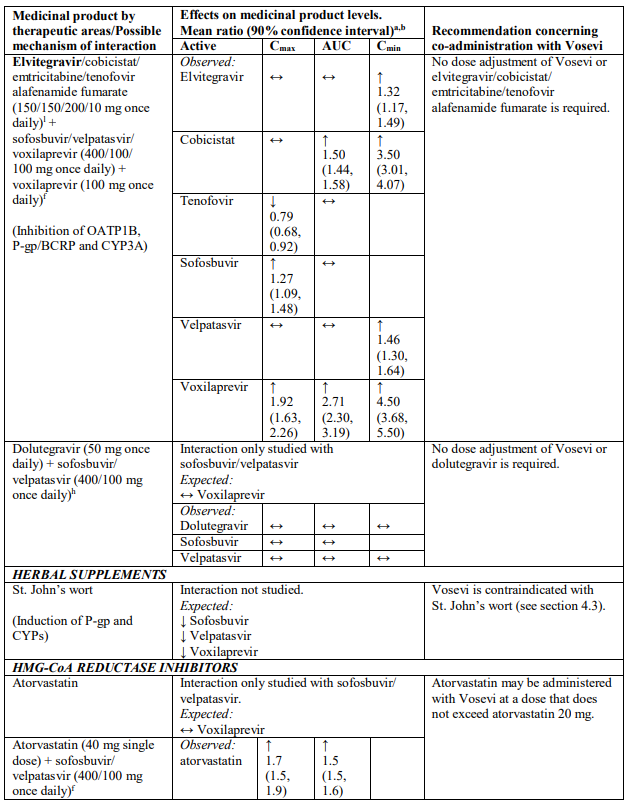
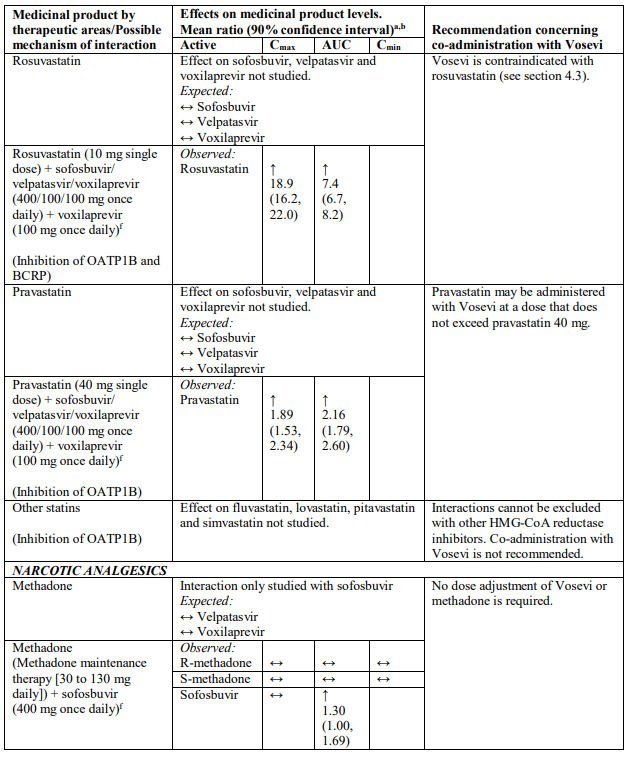
Interactions between Vosevi and other medicinal products
Table 2 provides a listing of established or potentially clinically significant medicinal product interactions (where 90% confidence interval [CI] of the geometric least-squares mean [GLSM] ratio were within “↔”, extended above “↑”, or extended below “↓” the predetermined interaction boundaries). The medicinal product interactions described are based on studies conducted with either sofosbuvir/velpatasvir/voxilaprevir, its components (sofosbuvir, velpatasvir, and/or voxilaprevir), or are predicted medicinal product interactions that may occur with Vosevi. The table is not all-inclusive.
Table 2. Interactions between Vosevi and other medicinal products:
a Mean ratio (90% CI) of co-administered drug pharmacokinetics of study medicinal products alone or in combination. No effect = 1.00.
b All interaction studies conducted in healthy volunteers.
c Lack of pharmacokinetics interaction lower bound 70%.
d These are medicinal products within class where similar interactions could be predicted.
e Bioequivalence/Equivalence boundary 80-125%.
f Lack of pharmacokinetics interaction bounds 70-143%.
g Administered as efavirenz, emtricitabine and tenofovir DF fixed-dose combination.
h Administered as sofosbuvir, velpatasvir fixed-dose combination.
i Administered as emtricitabine, rilpivirine, and tenofovir alafenamide fixed-dose combination.
j Administered as emtricitabine, tenofovir disoproxil fumarate fixed-dose combination.
k Lack of pharmacokinetics interaction bounds 50-200%.
l Administered as elvitegravir, cobicistat, emtricitabine and tenofovir alafenamide fixed-dose combination.
4.6. Fertility, pregnancy and lactation
Pregnancy
There are no or limited amount of data (less than 300 pregnancy outcomes) from the use of sofosbuvir, velpatasvir, voxilaprevir or Vosevi in pregnant women.
Sofosbuvir
Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity (see section 5.3).
It has not been possible to fully estimate exposure margins achieved for sofosbuvir in the rat relative to the exposure in humans at the recommended clinical dose (see section 5.3).
Velpatasvir
Animal studies have shown a possible link to reproductive toxicity (see section 5.3).
Voxilaprevir
Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity (see section 5.3).
As a precautionary measure, Vosevi use is not recommended during pregnancy.
Breast-feeding
It is unknown whether sofosbuvir, metabolites of sofosbuvir, velpatasvir or voxilaprevir are excreted in human milk.
Available pharmacokinetic data in animals have shown excretion of velpatasvir and metabolites of sofosbuvir in milk. When administered to lactating rats, voxilaprevir was detected in the plasma of nursing pups.
A risk to the newborns/infants cannot be excluded. Therefore, Vosevi should not be used during breast-feeding.
Fertility
No human data on the effect of Vosevi on fertility are available. Animal studies do not indicate harmful effects of sofosbuvir, velpatasvir or voxilaprevir on fertility.
4.7. Effects on ability to drive and use machines
Vosevi has no or negligible influence on the ability to drive and use machines.
4.8. Undesirable effects
Summary of the safety profile
In Phase 2 and 3 clinical studies, the proportion of patients who permanently discontinued treatment due to adverse reactions was 0.1% for patients receiving sofosbuvir/velpatasvir/voxilaprevir for 8 weeks. There were no patients receiving sofosbuvir/velpatasvir/voxilaprevir for 12 weeks who permanently discontinued treatment due to adverse reactions in the Phase 2 and 3 pivotal clinical studies.
Tabulated summary of adverse reactions
Assessment of adverse reactions for Vosevi is based on safety data from clinical studies and postmarketing experience. All adverse reactions are presented in Table 3. The adverse reactions are listed below by system organ class and frequency. Frequencies are defined as follows: very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1000 to <1/100); rare (≥1/10,000 to <1/1000) or very rare (<1/10,000).
Table 3. Adverse reactions identified with Vosevi:
| Frequency | Adverse reaction |
|---|---|
| Nervous system disorders | |
| Very common | headache |
| Gastrointestinal disorders | |
| Very common | diarrhoea, nausea |
| Common | abdominal pain, decreased appetite, vomiting |
| Skin and subcutaneous tissue disorders | |
| Common | rash |
| Uncommon | angioedemaa |
| Musculoskeletal and connective tissue disorders | |
| Common | myalgia |
| Uncommon | muscle spasm |
| Laboratory investigations | |
| Common | total bilirubin increased |
a Adverse reaction identified through post-marketing surveillance for sofosbuvir/velpatasvir-containing products.
Description of selected adverse reactions
Cardiac arrhythmias
Cases of severe bradycardia and heart block have been observed when sofosbuvir containing regimens are used in combination with amiodarone and/or other medicinal products that lower heart rate (see sections 4.4 and 4.5).
Skin disorders
Frequency not known: Stevens-Johnson syndrome
Laboratory abnormalities
Total bilirubin
In the Phase 3 studies increases in total bilirubin less than or equal to 1.5 x the upper limit of normal were observed in 4% of patients without cirrhosis and 10% of patients with compensated cirrhosis, due to inhibition of OATP1B1 and OATP1B3 by voxilaprevir. Total bilirubin levels decreased after completing Vosevi treatment.
Patients with renal impairment
The safety of sofosbuvir in a fixed dose combination with either ledipasvir or velpatasvir has been studied in 154 patients with ESRD requiring dialysis (Study 4062 and Study 4063). In this setting, exposure of sofosbuvir metabolite GS-331007 is 20-fold increased, exceeding levels where adverse reactions have been observed in preclinical studies. In this limited clinical safety data set, the rate of adverse events and deaths was not clearly elevated from what is expected in ESRD patients.
Paediatric population
The safety assessment of Vosevi in paediatric patients aged 12 years and older is based on data from 21 DAA-naïve patients with genotype 1, 2, 3, or 4 HCV infection (without cirrhosis) who were treated with Vosevi for 8 weeks in a Phase 2, open-label clinical study (study 1175). The adverse reactions observed were consistent with those observed in clinical studies of Vosevi in adults.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.