VOYDEYA Film-coated tablet Ref.[110089] Active ingredients: Danicopan
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Alexion Europe SAS, 103-105 rue Anatole France, 92300 Levallois-Perret, FRANCE
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Immunosuppressants, Complement inhibitors
ATC code: L04AJ09
Mechanism of action
Danicopan binds reversibly to complement factor D (FD) and acts as a selective inhibitor of FD function. By inhibiting FD, danicopan selectively blocks the activation of complement alternative pathway (AP), leading to prevention of the production of multiple effectors, that include C3 fragments, after AP activation. The 2 other complement pathways (classical and lectin) remain active. Danicopan's inhibitory effect on AP activation inhibits the deposition of C3 fragments on PNH red blood cells; such deposition is a key cause of the EVH which can become clinically significant in a small subset of patients with PNH on a C5 inhibitor. Maintenance of C5 inhibition controls the lifethreatening pathophysiological consequences of terminal complement activation underlying PNH.
Pharmacodynamic effects
In a clinical trial in patients with PNH with clinically significant EVH treated with ravulizumab or eculizumab, danicopan demonstrated the expected inhibition of AP activity, reduction of plasma Bb (a cleaved product of complement factor B by FD) level, as well as decreased C3 fragment deposition on circulating PNH red blood cells.
Cardiac electrophysiology
Single oral doses of danicopan administered at 400 mg, 800 mg, or 1 200 mg did not prolong QTc interval. There were no categorical alerts of concern regarding electrocardiogram intervals or wave form abnormalities.
Clinical efficacy and safety
The efficacy and safety of danicopan in adult patients with PNH who have clinically significant EVH were assessed in a multiple-region, randomised, double-blind, placebo-controlled, phase 3 study (ALXN2040-PNH-301). The study enrolled 86 patients with PNH who had been treated with a stable dose of ravulizumab or eculizumab for at least the previous 6 months and had anaemia (haemoglobin [Hgb] ≤9.5 g/dL [5.9 mmol/L]) with absolute reticulocyte count ≥120 × 109/L, with or without transfusion support.
Danicopan was administered in accordance with the recommended dosing described in section 4.2 (150 mg three times a day, and up to a maximum of 200 mg three times a day depending on the clinical response).
Patients were evaluated for history of vaccination and had to be vaccinated against meningococcal infection prior to or at the time of initiating treatment with danicopan if vaccination status within 3 years could not verified.
Patients were randomised to danicopan or placebo three times a day in a 2:1 ratio for 12 weeks in addition to background ravulizumab or eculizumab treatment in both groups. After week 12, all patients received danicopan as an add-on to their background ravulizumab or eculizumab treatment up to week 24. At the end of the treatment periods (week 24), patients were offered to enter a long-term extension (LTE) period and continued to receive danicopan with background ravulizumab or eculizumab.
Demographic or baseline characteristics were generally balanced between treatment groups. PNH medical history was similar between the treatment group and the placebo control group. The mean age at baseline was 52.8 years and the majority of patients were female (62.8%). Mean haemoglobin levels at baseline were 7.75 g/dL [4.81 mmol/L] and mean reticulocyte counts were 239.40 × 109/L. Within 24 weeks prior to the first dose, 76 patients (88.4%) had pRBC/whole blood transfusions and the mean number of transfusion instances was 2.6. Mean LDH levels were 298.13 U/L and mean FACIT-Fatigue scores were 33.24. The study enrolled 51 patients (59.3%) on ravulizumab and 35 patients (40.7%) on eculizumab.
The primary endpoint was the change in Hgb level from baseline to week 12. Secondary endpoints were the proportion of patients with Hgb increase of ≥2 g/dL [1.2 mmol/L] at week 12 in the absence of transfusions, the proportion of patients with transfusion avoidance through week 12, the change from baseline in Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scores at week 12, and change from baseline in absolute reticulocyte count at week 12. Transfusion avoidance was considered as achieved only by the patients who did not receive a transfusion and did not meet the protocol specified guidelines for transfusion from baseline through 12-week treatment period 1. The primary evidence for efficacy analysis is based on a pre-specified analysis performed when the first 63 randomised participants reached the end (either completed or discontinued) of the 12-week treatment period 1. Danicopan as an add-on to ravulizumab or eculizumab was superior to placebo as an add-on to ravulizumab or eculizumab for the primary endpoint and resulted in a statistically significant increase in Hgb from baseline to week 12. The LS mean change in Hgb from baseline was 2.94 g/dL [1.82 mmol/L] in the danicopan group compared with 0.50 g/dL [0.31 mmol/L] in the placebo group. The treatment group difference was 2.44 g/dL [1.51 mmol/L] (95% CI: 1.69 [1.05], 3.20 [1.99]); p<0.0001). Danicopan also achieved statistically significant improvement compared to placebo for all 4 secondary endpoints: proportion of patients with Hgb increase of ≥2 g/dL [1.2 mmol/L] in the absence of transfusion (59.5% vs. 0%, treatment difference: 46.9 [95% CI: 29.2, 64.7]; p<0.0001), proportion of patients with transfusion avoidance (83.3% vs. 38.1%, treatment difference: 41.7 [95% CI: 22.7, 60.8]; p=0.0004), change in FACIT-Fatigue score (7.97 vs. 1.85, treatment difference: 6.12 [95% CI: 2.33, 9.91]; p=0.0021) and change in absolute reticulocyte count (-83.8 vs. 3.5, treatment difference: -87.2 [95% CI: -117.7, -56.7]; p<0.0001).
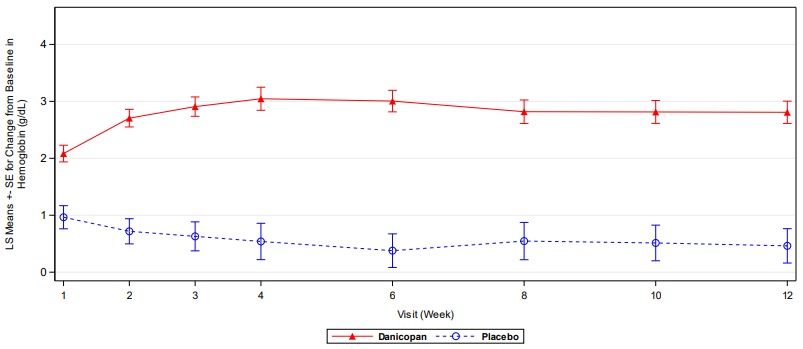
Supplemental results at week 12 based on all randomised patients (N=86) are consistent with those from the primary efficacy analysis (N=63). Danicopan as an add-on to ravulizumab or eculizumab was superior to placebo as an add-on to ravulizumab or eculizumab for the primary endpoint and resulted in a statistically significant increase in Hgb from baseline to week 12 (see Table 2 and Figure 1). Danicopan also achieved statistically significant improvement compared to placebo for all 4 secondary endpoints (see Table 2).
During the 12-week treatment period 1, 14 of 57 (24.6%) patients in the danicopan add-on group were dose escalated from 150 mg to 200 mg three times a day. Four patients (2 randomised to danicopan and 2 randomised to placebo) discontinued treatment during treatment period 1. There were no discontinuations due to haemolysis.
Table 2. Analysis of primary and secondary endpoints at week 12 (all randomised patients):
| Danicopan (add-on with ravulizumab or eculizumab) N=57 | Placebo (add-on with ravulizumab or eculizumab) N=29 | |
|---|---|---|
| Change in haemoglobin level (primary endpoint) | ||
| Mean change from baseline to week 12 (g/dL [mmol/L]) | 2.81 [1.74] | 0.46 [0.29] |
| Treatment difference* (95% CI) | 2.35 [1.46] (1.63 [1.01], 3.06 [1.90]) | |
| Proportion of patients with haemoglobin increase of ≥2 g/dL [1.2 mmol/L] in the absence of transfusion | ||
| At week 12 (%) | 54.4 | 0 |
| Treatment difference** (95% CI) | 47.5 (32.6, 62.4) | |
| Proportion of patients with transfusion avoidance | ||
| Through 12-week treatment period (%) | 78.9 | 27.6 |
| Treatment difference** (95% CI) | 48.4 (31.8, 64.9) | |
| Change in FACIT-Fatigue score | ||
| Mean change from baseline to week 12 | 8.10 | 2.38 |
| Treatment difference* (95% CI) | 5.72 (2.62, 8.83) | |
| Change in absolute reticulocyte count | ||
| Mean change from baseline to week 12 (109/L) | -92.6 | -0.9 |
| Treatment difference* (95% CI) | -91.6 (-120.0, -63.3) | |
* Based on mixed-effect model for repeated measures.
** Difference in rates and associated 95% CI are calculated using Miettinen and Nurminen method adjusting for stratification factors.
Abbreviations: CI = confidence interval; FACIT = Functional Assessment of Chronic Illness Therapy
Figure 1. Mean change in haemoglobin level from baseline to week 12 (all randomised patients):
Results at week 24 were consistent with those at week 12 and support maintenance of the effect. Among the 55 patients with PNH who received danicopan for 24 weeks, the LS mean change in Hgb from baseline to week 24 was 2.95 g/dL [1.83 mmol/L] (95% CI: 2.42 [1.50], 3.48 [2.16]), 69.1% maintained transfusion avoidance through week 24 and 41.8% had a Hgb increase of ≥2 g/dL [1.2 mmol/L] in the absence of transfusion at week 24. These patients also had consistent improvement in FACIT-Fatigue scores that was maintained through 24 weeks, the mean change from baseline was 6.19 (95% CI: 4.10, 8.29).
Efficacy results up to week 72 are consistent with those at week 12 and week 24 and support durability and maintenance of the effect over time. In patients who received danicopan for 72 weeks (N=16) the mean change in Hgb from baseline to week 72 was 2.99 g/dL [1.86 mmol/L].
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with Voydeya in one or more subsets of the paediatric population in the treatment of PNH (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Absorption
Danicopan is rapidly absorbed after oral dosing, with mean time to maximum observed concentration occurring at about 3 hours post dose. Over the dose range of 200 mg to 800 mg, Cmax increased in a less than dose-proportional manner, likely due to solubility-limited absorption. When danicopan was administered with a high-fat meal, AUC and Cmax were approximately 25%, and 93% higher, respectively, compared to the fasted state. Median Tmax was comparable when danicopan was administered in the non-fasted or fasted state at approximately 3.0 and 2.5 hours, respectively (see section 4.2).
Danicopan is highly permeable and a P-gp substrate in vitro but with low efflux ratio. The oral exposure of danicopan does not appear to be affected by P-gp efflux in the gastrointestinal tract. Danicopan is not a substrate of BCRP, OATP1B1, or OATP1B3.
Distribution
Danicopan is highly bound to human plasma proteins (91.5% to 94.3%) and is mainly distributed in plasma with a ratio of whole blood to plasma mean AUC0-∞ of 0.545. Danicopan plasma concentrations appeared to decline in a biphasic manner after Tmax. The estimated oral apparent volume of distribution for a 75 kg person using the population-PK model was 168 L for Vc/F and 234 L for Vp/F (402 L total), suggesting a moderate distribution of danicopan to peripheral tissue.
Biotransformation
Danicopan is extensively metabolised (96%) after oral dosing via oxidation, reduction, and hydrolysis pathways, with amide hydrolysis identified as the major pathway of elimination. Metabolism by CYP-mediated mechanisms is minimal.
Elimination
Following oral administration, the principal route of elimination is in the faeces (approximately 69% of the administered dose, compared to approximately 25% of the administered dose in urine). In the population pharmacokinetic (PK) analysis in patients with PNH who have clinically significant EVH, the t½ has an estimated mean value of 7.91 hours.
Special populations
No clinically significant differences in the pharmacokinetics of danicopan were observed based on sex, age, or race based on population PK assessment.
Renal impairment
Following oral administration of danicopan 200 mg in subjects with severe renal impairment (eGFR <30 mL/min/1.73 m²), the extent of danicopan exposure (AUC) increased by approximately 50% as compared to subjects with normal renal function. Renal excretion is not the major route for clearing danicopan from the body, even in subjects with normal renal function (see section 4.2).
Hepatic impairment
No significant difference in danicopan exposure is observed in subjects with moderate hepatic impairment (Child-Pugh Class B) as compared to subjects with normal hepatic function (see section 4.2). Studies have not been conducted in patients with severe hepatic impairment (Child-Pugh Class C).
5.3. Preclinical safety data
In the 6-month toxicity study in rats (species not pharmacologically sensitive to danicopan), hypertrophy in liver, thyroid and adrenal gland was observed at doses of 1000 mg/kg/day (~26-fold above human exposure at 200 mg three times a day based on AUC).
In the 9-month toxicity study in dogs, dose of 150 mg/kg/day was not tolerated. Target organ effects were observed in the liver consistent with hepatobiliary cholestasis and included bile duct hypertrophy/hyperplasia and pigment accumulation in Kupffer cell and hepatocyte, consistent with bile pigment. Increases in AST, ALT, ALP, GGT, and TBIL correlated with histological findings in the liver. Hypertrophy/hyperplasia of the bile duct was observed in males at doses greater than or equal to 75 mg/kg/day (~5-fold above human exposure at 200 mg three times a day based on AUC). However, the findings at the dose of 75 mg/kg/day were less in severity and magnitude and did not have correlative clinical pathology findings.
Genotoxicity/carcinogenicity
Danicopan was not genotoxic in the Ames bacterial reverse mutation assay, in vitro micronucleus assay in human peripheral blood lymphocytes or in the in vivo micronucleus assay in rats.
Danicopan was not carcinogenic in the 6-month carcinogenicity study in TgRasH2 mice and in the 2-year rat carcinogenicity study. However, in the rat study a higher incidence of endometrial epithelium neoplasmas at the highest dose of 500 mg/kg/day compared to control animals was observed although the rat strain can have a high background incidence of endometrial carcinomas. The clinical relevance of this finding is unknown.
Reproductive/developmental toxicity
In the fertility and early embryonic development study in rabbits, reduced male and female reproductive performance was observed at 500 mg/kg/day, a dose associated at poor tolerability. The NOAEL for male and female reproductive toxicity was considered to be 250 mg/kg/day (7.2- and 8.8-fold above the human exposure).
In the pre- and post-natal development study in rabbits, in the F1 males, a decrease (19, 20 and 18%) in cauda epididymal sperm concentration relative to controls was observed in all dose groups (50, 125 and 250 mg/kg/day, respectively), being statistically significant only in the low and mid dose groups. This did not impact the reproductive capability of the F1 generation.
There were no effects on early embryonic development and foetal development in rabbits up to mean maternal systemic exposure ~20-fold above human exposure or during post-natal development. In the rats, there were no effects on embryo-foetal development up to maternal exposure ~30-fold above the human exposure at 200 mg three times a day.
Excretion in milk
Danicopan was excreted into the milk of lactating rabbits following oral administration from lactation Day 4 to 10, with milk concentrations approximately 5 and 3.5 times higher compared to maternal plasma concentrations at 50 and 250 mg/kg/day, respectively.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.