VUMERITY Gastro-resistant hard capsule Ref.[28088] Active ingredients: Diroximel fumarate
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Biogen Netherlands B.V., Prins Mauritslaan 13, 1171 LP Badhoevedorp, The Netherlands
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Immunosuppressants, other immunosuppressants
ATC code: L04AX09
Mechanism of action
The mechanism by which diroximel fumarate exerts therapeutic effects in MS is not fully understood. Diroximel fumarate acts via the major active metabolite, monomethyl fumarate. Preclinical studies indicate that the pharmacodynamic responses of monomethyl fumarate appears to be mediated, at least in part, through activation of the Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) transcriptional pathway. Dimethyl fumarate has been shown to up regulate Nrf2-dependent antioxidant genes in patients.
Pharmacodynamic effects
Effects on Immune System
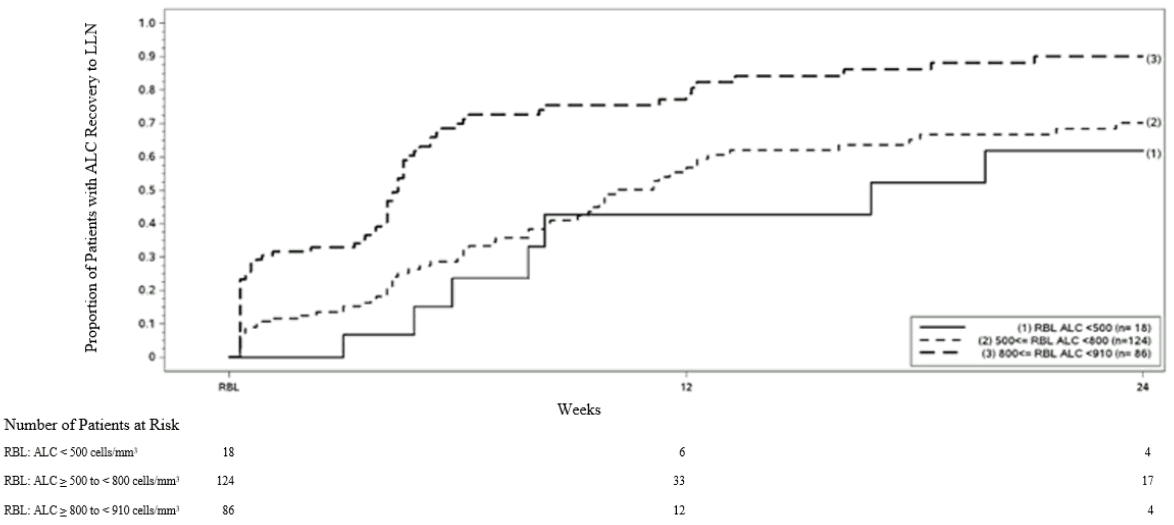
In clinical studies, dimethyl fumarate demonstrated anti-inflammatory and immunomodulatory properties. Dimethyl fumarate and monomethyl fumarate (the active metabolite of diroximel fumarate and dimethyl fumarate) significantly reduce immune cell activation and subsequent release of pro-inflammatory cytokines in response to inflammatory stimuli and moreover affect lymphocyte phenotypes through a down-regulation of pro-inflammatory cytokine profiles (TH1, TH17), and biased towards anti-inflammatory production (TH2). In phase 3 studies in MS patients (DEFINE, CONFIRM and ENDORSE), upon treatment with dimethyl fumarate mean lymphocyte counts decreased on average by approximately 30% of their baseline value over the first year with a subsequent plateau. In these studies, patients who discontinued dimethyl fumarate therapy with lymphocyte counts below the lower limit of normal (LLN, 910 cells/mm³) were monitored for recovery of lymphocyte counts to the LLN.
Figure 1 shows the proportion of patients estimated to reach the LLN based on the Kaplan-Meier method without prolonged severe lymphopenia. The recovery baseline (RBL) was defined as the last on-treatment ALC prior to dimethyl fumarate discontinuation. The estimated proportion of patients recovering to LLN (ALC ≥0.9 × 109/L) at Week 12 and Week 24, who had mild, moderate, or severe lymphopenia at RBL are presented in Table 2, Table 3, and Table 4 with 95% pointwise confidence intervals. The standard error of the Kaplan-Meier estimator of the survival function is computed using Greenwood’s formula.
Figure 1. Kaplan-Meier Method; Proportion of Patients with Recovery to ≥ 910 cells/mm³ LLN from the Recovery Baseline (RBL):
Table 2. Kaplan-Meier Method; Proportion of patients estimated to reach LLN, mild lymphopenia at the recovery baseline (RBL), excluding patients with prolonged severe lymphopenia:
| Number of patients with mild lymphopeniaa at risk | Baseline N=86 | Week 12 N=12 | Week 24 N=4 |
|---|---|---|---|
| Proportion reaching LLN (95% CI) | 0.81 (0.71, 0.89) | 0.90 (0.81, 0.96) |
a Patients with ALC <910 and ≥800 cells/mm³ at RBL, excluding patients with prolonged severe lymphopenia.
Table 3. Kaplan-Meier Method; Proportion of patients estimated to reach LLN, moderatelymphopenia at the recovery baseline (RBL), excluding patients with prolonged severe lymphopenia:
| Number of patients with moderate lymphopeniaa at risk | Baseline N=124 | Week 12 N=33 | Week 24 N=17 |
|---|---|---|---|
| Proportion reaching LLN (95% CI) | 0.57 (0.46, 0.67) | 0.70 (0.60, 0.80) |
a Patients with ALC <800 and ≥500 cells/mm³ at RBL, excluding patients with prolonged severe lymphopenia.
Table 4. Kaplan-Meier Method; Proportion of patients estimated to reach LLN, severe lymphopenia at the recovery baseline (RBL), excluding patients with prolonged severe lymphopenia:
| Number of patients with severe lymphopeniaa at risk | Baseline N=18 | Week 12 N=6 | Week 24 N=4 |
|---|---|---|---|
| Proportion reaching LLN (95% CI) | 0.43 (0.20, 0.75) | 0.62 (0.35, 0.88) |
a Patients with ALC <500 cells/mm³ at RBL, excluding patients with prolonged severe lymphopenia.
Clinical efficacy and safety
Diroximel fumarate and dimethyl fumarate are rapidly metabolised by esterases before they reach the systemic circulation to the same active metabolite, monomethyl fumarate, upon oral administration. The PK comparability of diroximel fumarate to dimethyl fumarate through the analysis of monomethyl fumarate exposure has been demonstrated (see section 5.2), thus efficacy profiles are expected to be similar.
Clinical studies with dimethyl fumarate
Two, 2-year, randomised, double-blind, placebo-controlled studies (DEFINE with 1,234 patients and CONFIRM with 1,417 patients) of patients with RRMS were performed. Patients with progressive forms of MS were not included in these studies.
Efficacy (see table below) and safety were demonstrated in patients with Expanded Disability Status Scale (EDSS) scores ranging from 0 to 5 inclusive, who had experienced at least 1 relapse during the year prior to randomisation, or, in the 6 weeks before randomisation had a brain Magnetic Resonance Imaging (MRI) demonstrating at least one gadolinium-enhancing (Gd+) lesion. Study CONFIRM contained a rater-blinded (i.e. study physician/investigator assessing the response to study treatment was blinded) reference comparator of glatiramer acetate.
In DEFINE, patients had the following median baseline characteristics: age 39 years, disease duration 7.0 years, EDSS score 2.0. In addition, 16% of patients had an EDSS score >3.5, 28% had ≥2 relapses in the prior year and 42% had previously received other approved MS treatments. In the MRI cohort 36% of patients entering the study had Gd+ lesions at baseline (mean number of Gd+ lesions 1.4).
In CONFIRM, patients had the following median baseline characteristics: age 37 years, disease duration 6.0 years, EDSS score 2.5. In addition, 17% of patients had an EDSS score >3.5, 32% had ≥2 relapses in the prior year and 30% had previously received other approved MS treatments. In the MRI cohort 45% of patients entering the study had Gd+ lesions at baseline (mean number of Gd+ lesions 2.4).
Compared to placebo, patients treated with dimethyl fumarate had a clinically meaningful and statistically significant reduction on: the primary endpoint in study DEFINE, proportion of patients relapsed at 2 years; and the primary endpoint in study CONFIRM, annualised relapse rate (ARR) at 2 years.
The ARR for glatiramer acetate and placebo was 0.286 and 0.401 respectively in study CONFIRM, corresponding to a reduction of 29% (p=0.013).
| DEFINE | CONFIRM | ||||
|---|---|---|---|---|---|
| Placebo | dimethyl fumarate 240 mg twice a day | Placebo | dimethyl fumarate 240 mg twice a day | Glatiramer acetate | |
| Clinical Endpointsa | |||||
| No. patients | 408 | 410 | 363 | 359 | 350 |
| Annualised relapse rate | 0.364 | 0.172*** | 0.401 | 0.224*** | 0.286* |
| Rate ratio (95% CI) | 0.47 (0.37, 0.61) | 0.56 (0.42, 0.74) | 0.71 (0.55, 0.93) | ||
| Proportion relapsed | 0.461 | 0.270*** | 0.410 | 0.291** | 0.321** |
| Hazard ratio (95% CI) | 0.51 (0.40, 0.66) | 0.66 (0.51, 0.86) | 0.71 (0.55, 0.92) | ||
| Proportion with 12-week confirmed disability progression | 0.271 | 0.164** | 0.169 | 0.128# | 0.156# |
| Hazard ratio (95% CI) | 0.62 (0.44, 0.87) | 0.79 (0.52, 1.19) | 0.93 (0.63, 1.37) | ||
| Proportion with 24 week confirmed disability progression | 0.169 | 0.128# | 0.125 | 0.078# | 0.108# |
| Hazard ratio (95% CI) | 0.77 (0.52, 1.14) | 0.62 (0.37, 1.03) | 0.87 (0.55, 1.38) | ||
| MRI Endpointb | |||||
| No. patients | 165 | 152 | 144 | 147 | 161 |
| Mean (median) number of new or newly enlarging T2 lesions over 2 year | 16.5 (7.0) | 3.2 (1.0)*** | 19.9 (11.0) | 5.7 (2.0)*** | 9.6 (3.0)*** |
| Lesion mean ratio (95% CI) | 0.15 (0.10, 0.23) | 0.29 (0.21, 0.41) | 0.46 (0.33, 0.63) | ||
| Mean (median) number of Gd lesions at 2 years | 1.8 (0) | 0.1 (0)*** | 2.0 (0.0) | 0.5 (0.0)*** | 0.7 (0.0)** |
| Odds ratio (95% CI) | 0.10 (0.05, 0.22) | 0.26 (0.15, 0.46) | 0.39 (0.24, 0.65) | ||
| Mean (median) number of new T1 hypointense lesions over 2 years | 5.7 (2.0) | 2.0 (1.0)*** | 8.1 (4.0) | 3.8 (1.0)*** | 4.5 (2.0)** |
| Lesion mean ratio (95% CI) | 0.28 (0.20, 0.39) | 0.43 (0.30, 0.61) | 0.59 (0.42, 0.82) | ||
a All analyses of clinical endpoints were intent-to-treat; bMRI analysis used MRI cohort
* P-value <0.05; **P-value <0.01; ***P-value <0.0001; #not statistically significant
An open non-controlled 8-year extension study (ENDORSE) enrolled 1,736 eligible RRMS patients from the pivotal studies (DEFINE and CONFIRM). The primary objective of the study was to assess the long-term safety of dimethyl fumarate in patients with RRMS. Of the 1,736 patients, approximately half (909, 52%) were treated for 6 years or longer. 501 patients were continuously treated with dimethyl fumarate 240 mg twice daily across all 3 studies and 249 patients who were previously treated with placebo in studies DEFINE and CONFIRM received treatment 240 mg twice daily in study ENDORSE. Patients who received treatment twice daily continuously were treated for up to 12 years.
During study ENDORSE, more than half of all patients treated with dimethyl fumarate 240 mg twice daily did not have a relapse. For patients continuously treated twice daily across all 3 studies, the adjusted ARR was 0.187 (95% CI: 0.156, 0.224) in studies DEFINE and CONFIRM and 0.141 (95% CI: 0.119, 0.167) in study ENDORSE. For patients previously treated with placebo, the adjusted ARR decreased from 0.330 (95% CI: 0.266, 0.408) in studies DEFINE and CONFIRM to 0.149 (95% CI: 0.116, 0.190) in study ENDORSE.
In study ENDORSE, the majority of patients (>75%) did not have confirmed disability progression (measured as 6-month sustained disability progression). Pooled results from the three studies demonstrated dimethyl fumarate treated patients had consistent and low rates of confirmed disability progression with slight increase in mean EDSS scores across ENDORSE. MRI assessments (up to year 6, including 752 patients who had previously been included in the MRI cohort of studies DEFINE and CONFIRM showed that the majority of patients (approximately 90%) had no Gd-enhancing lesions. Over the 6 years, the annual adjusted mean number of new or newly enlarging T2 and new T1 lesions remained low.
Efficacy in patients with high disease activity
In Studies DEFINE and CONFIRM, consistent treatment effect on relapses in a subgroup of patients with high disease activity was observed, whilst the effect on time to 3-month sustained disability progression was not clearly established. Due to the design of the studies, high disease activity was defined as follows:
- Patients with 2 or more relapses in one year, and with one or more Gd-enhancing lesions on brain MRI (n=42 in DEFINE; n=51 in CONFIRM) or,
- Patients who have failed to respond to a full and adequate course (at least one year of treatment) of beta-interferon, having had at least 1 relapse in the previous year while on therapy, and at least 9 T2-hyperintense lesions in cranial MRI or at least 1 Gd-enhancing lesion, or patients having an unchanged or increased relapse rate in the prior year as compared to the previous 2 years (n=177 in DEFINE; n=141 in CONFIRM).
Clinical studies with Vumerity
The gastrointestinal tolerability of diroximel fumarate was evaluated in a randomised, multi-centre, phase 3 study (EVOLVE-MS-2) in 504 adult patients with RRMS. The study included a 5-week, double-blind treatment period with two treatment arms. Patients had a 1-week titration period and were randomised (1:1) to receive diroximel fumarate 462 mg twice daily (n=253) or dimethyl fumarate 240 mg twice daily (n=251). Patients had the following median baseline characteristics: age 44 years, disease duration 6.0 years and EDSS score 2.5. In this study, GI tolerability was investigated using the Individual GI Symptom and Impact Scale (IGISIS), which evaluated the incidence, intensity, onset, duration, and functional impact of five individual GI symptoms: nausea, vomiting, upper abdominal pain, lower abdominal pain, and diarrhoea.
Overall gastrointestinal adverse reactions were observed in 34.8% of diroximel fumarate-treated patients and in 49.0% of dimethyl fumarate-treated patients. Treatment discontinuations were in total 1.6% and 6.0%, for diroximel fumarate and dimethyl fumarate, respectively. The discontinuations for gastrointestinal tolerability reasons were 0.8% and 4.8%, for diroximel fumarate and dimethyl fumarate, respectively. Treatment-emergent gastrointestinal adverse reactions of ≥ 5% for diroximel fumarate and dimethyl fumarate, respectively, were diarrhoea (15.4% and 22.3%), nausea (14.6% and 20.7%), upper abdominal pain (6.7% and 15.5%), abdominal pain (6.3% and 9.6%), lower abdominal pain (5.9% and 6.8%), and vomiting (3.6% and 8.8%).
Paediatric population
The efficacy of Vumerity in paediatric patients has not been established.
The European Medicines Agency has deferred the obligation to submit the results of studies with Vumerity in one or more subsets of the paediatric population in the treatment of MS (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Orally administered diroximel fumarate undergoes rapid presystemic hydrolysis by esterases and is primarily converted to the active metabolite, monomethyl fumarate, and the major inactive metabolite HES. Diroximel fumarate is not quantifiable in plasma following oral administration. Therefore, all pharmacokinetic analyses related to diroximel fumarate were performed with plasma monomethyl fumarate concentrations. Pharmacokinetic data were obtained from 10 clinical studies with healthy volunteers, 2 studies with MS patients and population PK analyses. Pharmacokinetic assessment has demonstrated that the exposure of monomethyl fumarate after oral administration of 462 mg diroximel fumarate and 240 mg dimethyl fumarate in adults is bioequivalent; therefore, diroximel fumarate is expected to provide a similar overall efficacy and safety profile to dimethyl fumarate.
Absorption
The median Tmax of monomethyl fumarate is 2.5 to 3 hours. The peak plasma concentration (Cmax) and overall exposure (AUC) increased dose proportionally in the dose range studied (49 mg to 980 mg). Following administration of diroximel fumarate 462 mg twice a day in MS patients in EVOLVE-MS1, the mean Cmax of monomethyl fumarate was 2.11 mg/L. The mean AUClast after a morning dose was 4.15 mg.h/L. The mean steady state daily AUC (AUCss) of monomethyl fumarate was estimated to be 8.32 mg.h/L in MS patients.
Co-administration of diroximel fumarate with a high-fat, high-calorie meal did not affect the AUC of monomethyl fumarate but resulted in an approximately 44% reduction in Cmax compared to fasted state. The monomethyl fumarate Cmax with low-fat and medium-fat meals was reduced by approximately 12% and 25%, respectively.
Food does not have a clinically significant effect on exposure of monomethyl fumarate. Therefore, Vumerity may be taken with or without food (see section 4.2).
Distribution
The apparent volume of distribution (Vd) for monomethyl fumarate is between 72 L and 83 L in healthy subjects after administration of diroximel fumarate. Human plasma protein binding of monomethyl fumarate was less than 25% and was not concentration dependent.
Biotransformation
In humans, diroximel fumarate is extensively metabolised by esterases, which are ubiquitous in the gastrointestinal tract, blood, and tissues, before it reaches the systemic circulation. Esterase metabolism of diroximel fumarate produces predominantly both monomethyl fumarate, the active metabolite, and HES, an inactive metabolite.
Further metabolism of monomethyl fumarate occurs through esterases followed by the tricarboxylic acid (TCA) cycle, with no involvement of the cytochrome P450 (CYP) system. Fumaric and citric acid, and glucose are the resulting metabolites of monomethyl fumarate in plasma.
Elimination
Monomethyl fumarate is mainly eliminated as carbon dioxide in the expired air with only trace amounts recovered in urine. The terminal half-life (t1/2) of monomethyl fumarate is approximately 1 hour, and no accumulation in monomethyl fumarate plasma exposures occurred with multiple doses of diroximel fumarate. In a study with dimethyl fumarate, exhalation of CO2 was determined to be the primary route of elimination accounting for approximately 60% of the dose. Renal and faecal elimination are secondary routes of elimination, accounting for 15.5% and 0.9% of the dose, respectively.
HES is eliminated from plasma with a t1/2 of 10.7 hours to 14.8 hours. HES is mainly eliminated in urine.
Linearity
Monomethyl fumarate exposure increases in an approximately dose proportional manner with single and multiple doses in the 49 to 980 mg dose range studied.
Pharmacokinetics in special patient groups
Body weight is the main covariate with monomethyl fumarate exposure increasing in Cmax and AUC in participants with lower body weight after administration of diroximel fumarate. No effect was seen on safety and efficacy measures evaluated in the clinical studies. Therefore, no dose adjustments based on body weight are required.
Gender and age did not have a statistically significant impact on Cmax and AUC of diroximel fumarate. The pharmacokinetics in patients aged 65 and over has not been studied.
Paediatric population
The pharmacokinetic profile of monomethyl fumarate after administration of diroximel fumarate has not been studied. The pharmacokinetic parameters of monomethyl fumarate after administration of diroximel fumarate are correlated to body weight. Therefore, it is anticipated that the same dose leads to a higher exposure in paediatric patients with lower body weight compared to adults. The pharmacokinetic profile of 240 mg dimethyl fumarate twice a day was evaluated in a small, open-label, uncontrolled study in patients with RRMS aged 13 to 17 years (n=21). The pharmacokinetics of dimethyl fumarate in these adolescent patients was similar with that previously observed in adult patients.
Race and ethnicity
Race and ethnicity have no effect on the pharmacokinetic profile of monomethyl fumarate or HES after administration of diroximel fumarate.
Renal impairment
In a study investigating the effect of renal impairment on the pharmacokinetic profile of diroximel fumarate, participants with mild (eGFR 60-89 mL/min/1.73cm³), moderate renal impairment (eGFR 30-59 mL/min/1.73cm³) or severe renal impairment (eGFR <30 mL/min/1.73cm³) had no clinically relevant changes in MMF exposure. However, HES exposure increased by 1.3-, 1.8-, and 2.7-fold with mild, moderate, and severe renal impairment, respectively (see section 4.8). There are no data available on long-term use of diroximel fumarate in patients with moderate or severe renal impairment (see sections 4.2 and 4.4).
Hepatic impairment
As diroximel fumarate and monomethyl fumarate are metabolised by esterases, without the involvement of the CYP450 system, evaluation of pharmacokinetics in individuals with hepatic impairment was not conducted (see section 4.2 and 4.4).
5.3. Preclinical safety data
Toxicology
Kidney toxicity in rats and monkeys included tubular degeneration/necrosis with regeneration, tubular hypertrophy and/or interstitial fibrosis, increased kidney weights, and changes in clinical pathology parameters (urine volume, specific gravity, and biomarkers of kidney injury). In chronic toxicology studies, adverse renal findings occurred at monomethyl fumarate exposure that equalled the AUC at the maximum recommended human dose (MRHD) of diroximel fumarate.
Gastrointestinal toxicity in mice and rats consisted of mucosal hyperplasia and hyperkeratosis in the non-glandular stomach (forestomach) and duodenum. In monkeys, the poor gastrointestinal tolerability was characterised by dose-dependent emesis/vomitus, stomach irritation, haemorrhage and inflammation as well as diarrhoea. These findings developed at monomethyl fumarate exposure at least 2x the AUC at the MRHD of diroximel fumarate.
Cardiac inflammation and necrosis was seen in three male rats in the 91-day toxicity study at monomethyl fumarate exposure that was 4 the AUC at the MRHD of diroximel fumarate. These cardiac findings were also detected in other toxicity studies in rats including untreated controls, but not in monkeys. These cardiac inflammations therefore likely represent the exacerbation of common background lesions in rats without human relevance.
Partially-reversible physeal dysplasia of proximal and distal femur and proximal tibia was seen in monkeys in the 91-day toxicity study at monomethyl fumarate exposure that was 15 the AUC at the MRHD of diroximel fumarate. Bone toxicity might be related to the pre-pupertal age of the monkeys, because bone development was also impaired in juvenile rats (see below), but not affected at lower doses in the chronic monkey study or in mature adult rats. The bone findings are of limited relevance for adult patients at the therapeutic dose.
Testicular toxicity consisting of minimal germinal epithelial degeneration, increased incidence of giant spermatids, slight decrease in spermatids in the tubular epithelium, and decrease in testes weight was observed in wild type littermates of rasH2 mice. These findings occurred at monomethyl fumarate exposure that was 15 the AUC at the MRHD of diroximel fumarate, indicating limited human relevance at the therapeutic dose.
Genotoxicity
In vitro and in vivo studies with diroximel fumarate did not provide evidence for a clinically relevant genotoxic potential.
Carcinogenesis
Diroximel fumarate was tested in a transgenic bioassay in transgenic rasH2 mice and a 2 year bioassay in rats. Diroximel fumarate was not carcinogenic in transgenic mice and in female rats, but increased the incidence of testicular Leydig cell adenomas at 150 mg/kg/day in male rats (monomethyl fumarate exposure was approximately 2 higher than the AUC at the MRHD). The relevance of these findings to human risk is unknown.
Reproduction and developmental toxicity
Diroximel fumarate did not impair male or female fertility in rats at monomethyl fumarate exposure that was approximately 7 the AUC at the MRHD of diroximel fumarate.
In rats administered diroximel fumarate orally during the period of organogenesis at doses of 40, 100 and 400 mg/kg/day lower fetal body weights and fetal skeletal ossification variations were observed at a maternally toxic diroximel fumarate dose of 400 mg/kg/day. The exposure at the NOAEL was approximately 2 the AUC of monomethyl fumarate at the MRHD of diroximel fumarate.
In rabbits administered diroximel fumarate orally throughout organogenesis at doses of 50, 150 and 350 mg/kg/day, increases in skeletal malformations (vertebral centra anomaly, severely malaligned sternebra[e] and vertebral anomaly with associated rib anomaly) were observed at ≥ 150 mg/kg/day. At 350 mg/kg/day, increases in skeletal variations, abortions, higher post-implantation loss and corresponding decreases in fetal viability also occurred, possibly associated with maternal toxicity. The exposure at the NOAEL was approximately 2 the AUC of monomethyl fumarate at the MRHD of diroximel fumarate. The relevance of the skeletal malformations for humans is currently unknown.
In a pre- and post-natal development study in pregnant rats administered diroximel fumarate at oral doses of 40, 100, or 400 mg/kg/day during gestation through delivery and lactation reduced maternal body weight/weight gains and food consumption associated with reduced pup birth weights and body weight/weight gains were observed. The exposure at the NOAEL was approximately 3 the AUC of monomethyl fumarate at the MRHD of diroximel fumarate.
Toxicity in juvenile animals
In a juvenile rat toxicity study, diroximel fumarate was administered orally from postnatal day (PND) 25 through PND 63, equivalent to approximately 2-3 years old through to puberty in humans. In addition to the target organ toxicities in the kidney and non-glandular stomach, adverse effects in the bone were observed including decreased femur size, mass and density and changes in bone geometry. A relation of the bone effects to lower body weight is possible, but the involvement of a direct effect cannot be excluded. The exposure at the NOAEL was approximately 1.4x the AUC of monomethyl fumarate at the MRHD for adult patients of diroximel fumarate. The bone findings are of limited relevance for adult patients. The relevance for paediatric patients is not known.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.