VYLOY Powder for concentrate for solution for infusion Ref.[112792] Active ingredients: Zolbetuximab
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Astellas Pharma Europe B.V., Sylviusweg 62, 2333 BE Leiden, The Netherlands
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, other monoclonal antibodies and antibody drug conjugates
ATC code: L01FX31
Mechanism of action
Zolbetuximab is a chimeric (mouse/human IgG1) monoclonal antibody directed against the tight junction molecule CLDN18.2. Nonclinical data suggest zolbetuximab binds selectively to cell lines transfected with CLDN18.2 or those that endogenously express CLDN18.2. Zolbetuximab depletes CLDN18.2-positive cells via antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). Cytotoxic medicinal products were shown to increase CLDN18.2 expression on human cancer cells and to improve zolbetuximab-induced ADCC and CDC activities.
Pharmacodynamic effects
Based on the exposure-response analyses of efficacy and safety in patients with locally advanced unresectable or metastatic HER2-negative gastric or GEJ adenocarcinoma whose tumours are CLDN18.2 positive, there are no anticipated clinically significant differences in efficacy or safety between zolbetuximab doses of 800/400 mg/m² every 2 weeks and 800/600 mg/m² every 3 weeks.
Immunogenicity
Based on a pooled analysis of data from two phase 3 studies, the overall immunogenicity incidence was 4.4% (21 of 479 total patients treated with zolbetuximab 800/600 mg/m² every 3 weeks in combination with mFOLFOX6/CAPOX were tested positive for anti-drug antibodies [ADAs]). Because of the low occurrence of ADAs, the effect of these antibodies on the pharmacokinetics, safety and/or effectiveness of zolbetuximab is unknown.
Clinical efficacy and safety
Gastric or GEJ adenocarcinoma
SPOTLIGHT (8951-CL-0301) and GLOW (8951-CL-0302)
The safety and efficacy of zolbetuximab in combination with chemotherapy was evaluated in two phase 3, double-blind, randomised, multicentre studies that enrolled 1072 patients whose tumours were CLDN18.2 positive, HER2-negative, with locally advanced unresectable or metastatic gastric or GEJ adenocarcinoma. CLDN18.2 positivity (defined as ≥75% of tumour cells demonstrating moderate to strong membranous CLDN18 staining) was determined by immunohistochemistry on gastric or GEJ tumour tissue specimens from all patients with the VENTANA CLDN18 (43-14A) RxDx Assay performed in a central laboratory.
Patients were randomised 1:1 to receive either zolbetuximab in combination with chemotherapy (n=283 in SPOTLIGHT, n=254 in GLOW) or placebo in combination with chemotherapy (n=282 in SPOTLIGHT, n=253 in GLOW). Zolbetuximab was administered intravenously at a loading dose of 800 mg/m² (Day 1 of cycle 1) followed by maintenance doses of 600 mg/m² every 3 weeks in combination with either mFOLFOX6 (oxaliplatin, folinic acid and fluorouracil), or CAPOX (oxaliplatin and capecitabine).
Patients in the SPOTLIGHT study received between 1-12 treatments of mFOLFOX6 [oxaliplatin 85 mg/m², folinic acid (leucovorin or local equivalent) 400 mg/m², fluorouracil 400 mg/m² given as a bolus and fluorouracil 2400 mg/m² given as a continuous infusion] administered on Days 1, 15 and 29 of a 42-day cycle. After 12 treatments, patients were allowed to continue treatment with zolbetuximab, 5-fluorouracil and folinic acid (leucovorin or local equivalent) at the discretion of the investigator, until progression of disease or unacceptable toxicity.
Patients in the GLOW study received between 1-8 treatments of CAPOX administered on Day 1 (oxaliplatin 130 mg/m²) and on Days 1 to 14 (capecitabine 1000 mg/m²) of a 21-day cycle. After 8 treatments of oxaliplatin, patients were allowed to continue treatment of zolbetuximab and capecitabine at the discretion of the investigator, until progression of disease or unacceptable toxicity.
Baseline characteristics were generally similar between studies, except for the proportion of Asian versus non-Asian patients in each study.
In the SPOTLIGHT study, the median age was 61 years (range: 20 to 86); 62% were male; 53% were Caucasian, 38% were Asian; 31% were from Asia and 69% were not from Asia. Patients had a baseline Eastern Cooperative Oncology Group (ECOG) performance status of 0 (43%) or 1 (57%). Patients had a mean body surface area of 1.7 m² (range: 1.1 to 2.5). The median time from diagnosis was 56 days (range: 2 to 5366); 36% of tumour types were diffuse, 24% were intestinal; 76% had gastric adenocarcinoma, 24% had GEJ adenocarcinoma; 16% had locally advanced disease and 84% had metastatic disease.
In the GLOW study, the median age was 60 years (range: 21 to 83); 62% were male; 37% were Caucasian, 63% were Asian; 62% were from Asia and 38% were not from Asia. Patients had a baseline ECOG performance status of 0 (43%) or 1 (57%). Patients had a mean body surface area of 1.7 m² (range: 1.1 to 2.3). The median time from diagnosis was 44 days (range: 2 to 6010); 37% of tumour types were diffuse, 15% were intestinal; 84% had gastric adenocarcinoma, 16% had GEJ adenocarcinoma; 12% had locally advanced disease and 88% had metastatic disease.
The primary efficacy outcome was progression-free survival (PFS) as assessed per RECIST v1.1 by an independent review committee (IRC). The key secondary efficacy outcome was overall survival (OS). Other secondary efficacy outcomes were objective response rate (ORR) and duration of response (DOR) as assessed per RECIST v1.1 by IRC.
In the primary analysis (final PFS and interim OS), the SPOTLIGHT study demonstrated a statistically significant benefit in PFS (as assessed by IRC) and OS for patients who received zolbetuximab in combination with mFOLFOX6 compared with patients who received placebo in combination with mFOLFOX6 treatment. The PFS HR was 0.751 (95% CI: 0.598, 0.942; 1-sided P = 0.0066) and the OS HR was 0.750 (95% CI: 0.601, 0.936; 1-sided P = 0.0053).
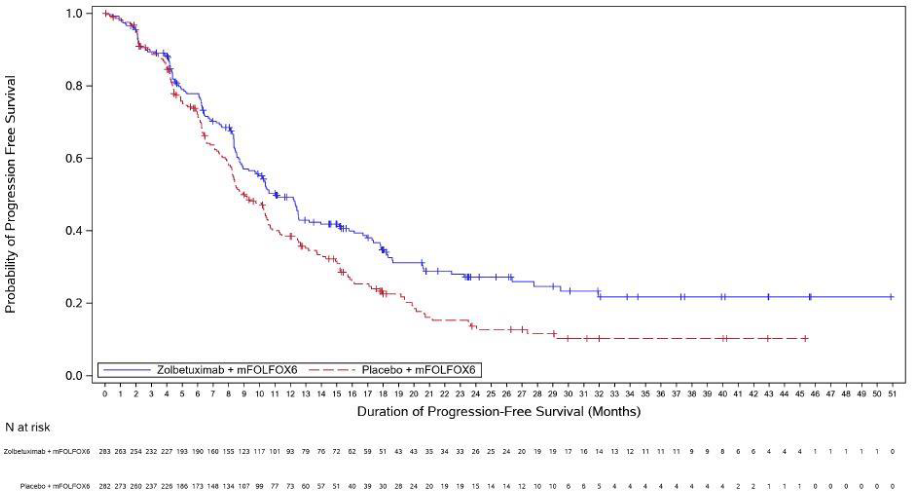
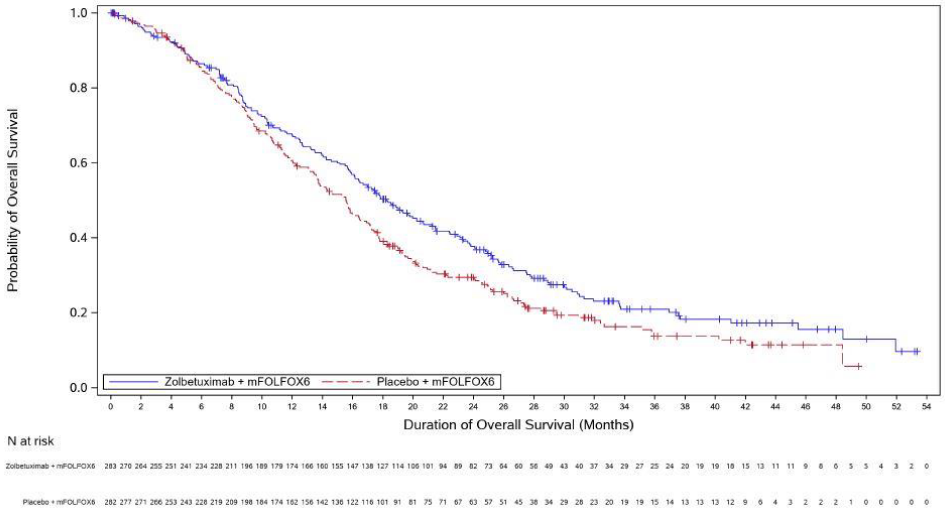
The updated PFS and final OS analysis for SPOTLIGHT are presented in table 5 and Figures 1-2 show the Kaplan- Meier curves.
In the primary analysis (final PFS and interim OS), the GLOW study demonstrated a statistically significant benefit in PFS (as assessed by IRC) and OS for patients who received zolbetuximab in combination with CAPOX compared with patients who received placebo in combination with CAPOX treatment. The PFS HR was 0.687 (95% CI: 0.544, 0.866; 1-sided P = 0.0007) and the OS HR was 0.771 (95% CI: 0.615, 0.965; 1-sided P = 0.0118).
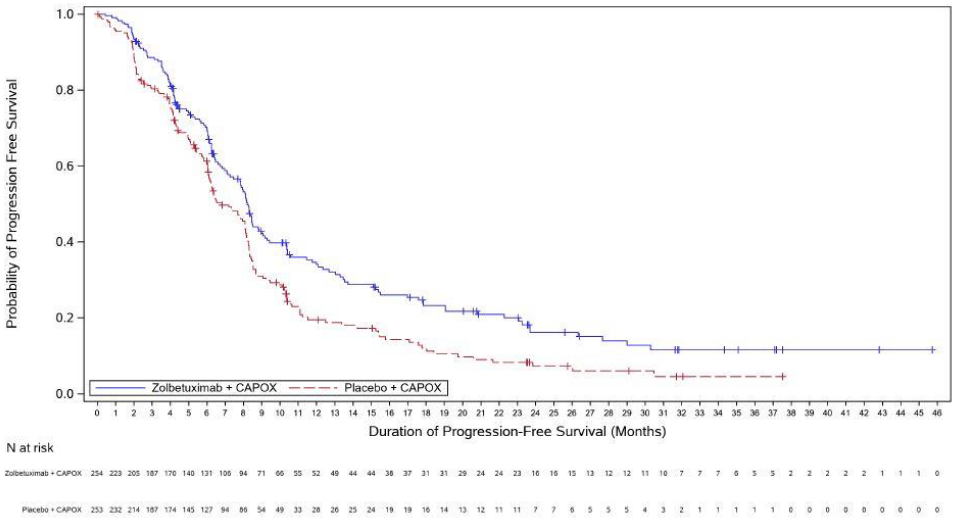
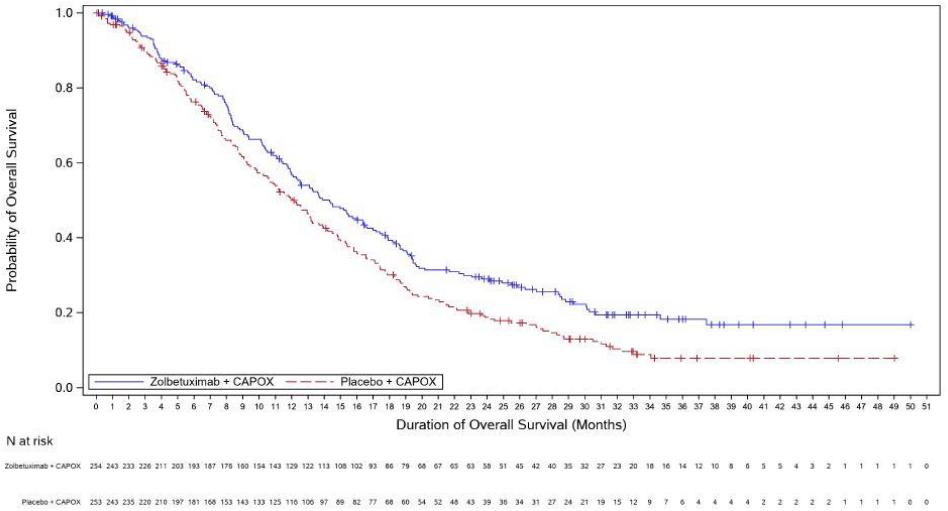
The updated PFS and final OS analysis for GLOW are presented in table 5 and Figures 3-4 show the Kaplan-Meier curves.
Table 5. Efficacy results in SPOTLIGHT and GLOW:
| Endpoint | SPOTLIGHTa | GLOWb | ||
|---|---|---|---|---|
| Zolbetuximab with mFOLFOX6 n=283 | Placebo with mFOLFOX6 n=282 | Zolbetuximab with CAPOX n=254 | Placebo with CAPOX n=253 | |
| Progression-free survival | ||||
| Number (%) of patients with events | 159 (56.2) | 187 (66.3) | 153 (60.2) | 182 (71.9) |
| Median in months (95% CI)c | 11.0 (9.7, 12.5) | 8.9 (8.2, 10.4) | 8.2 (7.3, 8.8) | 6.8 (6.1, 8.1) |
| Hazard ratio (95% CI)d,e | 0.734 (0.591, 0.910) | 0.689 (0.552, 0.860) | ||
| Overall survival | ||||
| Number (%) of patients with events | 197 (69.6) | 217 (77.0) | 180 (70.9) | 207 (81.8) |
| Median in months (95% CI)c | 18.2 (16.1, 20.6) | 15.6 (13.7, 16.9) | 14.3 (12.1, 16.4) | 12.2 (10.3, 13.7) |
| Hazard ratio (95% CI)d,e | 0.784 (0.644, 0.954) | 0.763 (0.622, 0.936) | ||
| Objective response rate (ORR), Duration of response (DOR) | ||||
| ORR () (95 CI)f | 48.1 (42.1, 54.1) | 47.5 (41.6, 53.5) | 42.5 (36.4, 48.9) | 39.1 (33.1, 45.4) |
| DOR Median in months (95% CI)f | 9.0 (7.5, 10.4) | 8.1 (6.5, 11.4) | 6.3 (5.4, 8.3) | 6.1 (4.4, 6.3) |
a SPOTLIGHT data cut-off: 08-Sep-2023, median follow-up time of zolbetuximab in combination with mFOLFOX6 arm was 18.0 months.
b GLOW data cut-off: 12-Jan-2024, median follow-up time of zolbetuximab in combination with CAPOX arm 20.6 months.
c Based on Kaplan-Meier estimate.
d Stratification factors were region, number of metastatic sites, prior gastrectomy from interactive response technology and study ID (SPOTLIGHT/GLOW).
e Based on Cox proportional hazards model with treatment, region, number of organs with metastatic sites, prior gastrectomy as the explanatory variables and study ID (SPOTLIGHT/GLOW).
f Based on IRC assessment and unconfirmed responses.
A combined efficacy analysis of SPOTLIGHT and GLOW of the final OS and updated PFS resulted in a median PFS (as assessed by IRC) of 9.2 months (95% CI: 8.4, 10.4) for zolbetuximab in combination with mFOLFOX6/CAPOX versus 8.2 months (95% CI: 7.6, 8.4) for placebo with mFOLFOX6/CAPOX [HR 0.712, 95% CI: 0.610, 0.831] and a median OS for zolbetuximab in combination with mFOLFOX6/CAPOX of 16.4 months (95% CI: 15.0, 17.9) versus 13.7 months (95% CI: 12.3, 15.3) for placebo with mFOLFOX6/CAPOX [HR 0.774, 95% CI: 0.672, 0.892].
Figure 1. Kaplan Meier plot of progression-free survival, SPOTLIGHT:
Figure 2. Kaplan Meier plot of overall survival, SPOTLIGHT:
Figure 3. Kaplan Meier plot of progression-free survival, GLOW:
Figure 4. Kaplan Meier plot of overall survival, GLOW:
Exploratory subgroup analyses of efficacy for SPOTLIGHT and GLOW showed a difference in PFS and OS for Caucasian versus Asian patients.
For SPOTLIGHT, in Caucasian patients this resulted in a PFS (as assessed by IRC) with a HR of 0.872 [95% CI: 0.653, 1.164] and an OS HR of 0.940 [95% CI: 0.718, 1.231] for zolbetuximab in combination with mFOLFOX6 versus placebo with mFOLFOX6. In Asian patients, this resulted in a PFS (as assessed by IRC) with a HR of 0.526 [95% CI: 0.354, 0.781] and an OS HR of 0.636 [95% CI: 0.450, 0.899] for zolbetuximab in combination with mFOLFOX6 versus placebo with mFOLFOX6. For GLOW, in Caucasian patients this resulted in a PFS (as assessed by IRC) with a HR of 0.891 [95% CI: 0.622, 1.276] and an OS HR of 0.805 [95% CI: 0.579, 1.120] for zolbetuximab in combination with CAPOX versus placebo with CAPOX. In Asian patients, this resulted in a PFS (as assessed by IRC) with a HR of 0.616 [95% CI: 0.467, 0.813] and an OS HR of 0.710 [95% CI: 0.549, 0.917] for zolbetuximab in combination with CAPOX versus placebo with CAPOX.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with zolbetuximab in all subsets of the paediatric population in gastric or GEJ adenocarcinoma (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Following intravenous administration, zolbetuximab exhibited dose-proportional pharmacokinetics at doses ranging from 33 mg/m² to 1000 mg/m². When administered at 800/600 mg/m² every 3 weeks, steady state was achieved by 24 weeks with a mean (SD) Cmax and AUCtau at 453 (82) μg/mL and 4125 (1169) day•μg/mL, respectively, based on a population pharmacokinetic analysis. When administered at 800/400 mg/m² every 2 weeks, steady state is expected to be achieved by 22 weeks with a mean (SD) Cmax and AUCtau at 359 (68) μg/mL and 2758 (779) day•μg/mL, respectively, based on a population pharmacokinetics analysis.
Distribution
The estimated mean steady state volume of distribution of zolbetuximab was 5.5 L.
Biotransformation
Zolbetuximab is expected to be catabolised into small peptides and amino acids.
Elimination
Zolbetuximab clearance (CL) decreased over time, with a maximal reduction from baseline values of 57.6% resulting in a population mean steady-state clearance (CLss) of 0.0117 L/h. The half-life of zolbetuximab ranged from 7.6 to 15.2 days during treatment.
Special populations
Elderly
Population pharmacokinetic analysis indicates that age [range: 22 to 83 years; 32.2% (230/714) were >65 years, 5.0% (36/714) were >75 years] did not have a clinically meaningful effect on the pharmacokinetics of zolbetuximab.
Race and gender
Based on the population pharmacokinetic analysis, no clinically significant differences in the pharmacokinetics of zolbetuximab were identified based on gender [62.3% male, 37.7% female] or race [50.1% Caucasian, 42.2% Asian, 4.2% Missing, 2.7% Others, and 0.8% Black].
Renal impairment
Based on the population pharmacokinetic analysis using data from clinical studies in patients with gastric or GEJ adenocarcinoma, no clinically significant differences in the pharmacokinetics of zolbetuximab were identified in patients with mild (CrCL ≥60 to <90 mL/min; n=298) to moderate (CrCL ≥30 to <60 mL/min; n=109) renal impairment based on CrCL estimated by the Cockcroft-Gault formula. Zolbetuximab has only been evaluated in a limited number of patients with severe renal impairment (CrCL ≥15 to <30 mL/min; n=1). The effect of severe renal impairment on the pharmacokinetics of zolbetuximab is unknown.
Hepatic impairment
Based on the population pharmacokinetic analysis using data from clinical studies in patients with gastric or GEJ adenocarcinoma, no clinically significant differences in the pharmacokinetics of zolbetuximab were identified in patients with mild hepatic impairment as measured by TB and AST (TB ≤ ULN and AST > ULN, or TB > 1 to 1.5 × ULN and any AST; n=108). Zolbetuximab has only been evaluated in a limited number of patients with moderate hepatic impairment (TB > 1.5 to 3 × ULN and any AST; n=4) and has not been evaluated in patients with severe hepatic impairment (TB > 3 to 10 × ULN and any AST). The effect of moderate or severe hepatic impairment on the pharmacokinetics of zolbetuximab is unknown.
5.3. Preclinical safety data
No studies in animals have been performed to evaluate carcinogenicity or mutagenicity.
No toxicity or other zolbetuximab-related adverse effects on the cardiovascular, respiratory or central nervous systems was observed in mice administered zolbetuximab for 13 weeks at systemic exposures up to 7.0-fold the human exposure at the recommended dose of 600 mg/m² (based on AUC) or in cynomolgus monkeys administered zolbetuximab for 4 weeks at systemic exposures up to 6.1-fold the human exposure at the recommended dose of 600 mg/m² (based on AUC).
In an embryo-foetal development toxicity study, where zolbetuximab was administered to pregnant mice during the period of organogenesis at systemic exposures up to approximately 6.2-fold the human exposure at the recommended dose of 600 mg/m² (based on AUC), zolbetuximab crossed the placental barrier. The resulting concentration of zolbetuximab in foetal serum at Day 18 of gestation was higher than that in the maternal serum at Day 16 of gestation. Zolbetuximab did not result in any external or visceral foetal abnormalities (malformations or variations).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.