YERVOY Concentrate for solution for infusion Ref.[8371] Active ingredients: Ipilimumab
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Bristol-Myers Squibb Pharma EEIG, Plaza 254, Blanchardstown Corporate Park 2, Dublin 15, D15 T867, Ireland
Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, monoclonal antibodies and antibody drug conjugates, other monoclonal antibodies and antibody drug conjugats
ATC code: L01FX04
Mechanism of action
Cytotoxic T-lymphocyte antigen-4 (CTLA-4) is a key regulator of T-cell activity. Ipilimumab is a CTLA-4 immune checkpoint inhibitor that blocks T-cell inhibitory signals induced by the CTLA-4 pathway, increasing the number of reactive T-effector cells which mobilize to mount a direct T-cell immune attack against tumour cells. CTLA-4 blockade can also reduce T-regulatory cell function, which may contribute to an anti-tumour immune response. Ipilimumab may selectively deplete T-regulatory cells at the tumour site, leading to an increase in the intratumoral T-effector/T-regulatory cell ratio which drives tumour cell death.
Pharmacodynamic effects
In patients with melanoma who received ipilimumab, the mean peripheral blood absolute lymphocyte counts (ALC) increased throughout the induction dosing period. In Phase 2 studies, this increase was dose-dependent. In MDX010-20 (see section 5.1), ipilimumab at 3 mg/kg with or without gp100 increased ALC throughout the induction dosing period, but no meaningful change in ALC was observed in the control group of patients who received an investigational gp100 peptide vaccine alone. In peripheral blood of patients with melanoma, a mean increase in the percent of activated HLA-DR+ CD4+ and CD8+ T cells was observed after treatment with ipilimumab, consistent with its mechanism of action. A mean increase in the percent of central memory (CCR7+ CD45RA-) CD4+ and CD8+ T cells and a smaller, but significant, mean increase in the percent of effector memory (CCR7- CD45RA-) CD8+ T cells also was observed after treatment with ipilimumab.
Clinical efficacy and safety
Ipilimumab in combination with nivolumab
For additional information on clinical efficacy and safety associated with the dosing recommendations of nivolumab when administered as monotherapy following combination therapy with ipilimumab, please refer to the nivolumab SmPC.
Based on modelling of dose/exposure efficacy and safety relationships, there are no clinically significant differences in efficacy and safety between a nivolumab dose of 240 mg every 2 weeks or 3 mg/kg every 2 weeks. Additionally, based on these relationships, there were no clinically significant differences between a nivolumab dose of 480 mg every 4 weeks or 3 mg/kg every 2 weeks in advanced melanoma and RCC.
Clinical trials with ipilimumab monotherapy
Melanoma
Overall survival (OS) advantage of ipilimumab at the recommended dose of 3 mg/kg in patients with previously-treated advanced (unresectable or metastatic) melanoma was demonstrated in a Phase 3 study (MDX010-20). Patients with ocular melanoma, primary CNS melanoma, active brain metastases, human immunodeficiency virus (HIV), hepatitis B, and hepatitis C were not included in the MDX010-20 clinical trial. Clinical trials excluded patients with ECOG performance status >1 and mucosal melanoma. Patients without liver metastasis who had a baseline AST >2.5 x ULN, patients with liver metastasis who had a baseline AST > 5 x ULN, and patients with a baseline total bilirubin ≥3 x ULN were also excluded.
For patients with a history of autoimmune disease, see also section 4.4.
MDX010-20
A Phase 3, double-blind study enrolled patients with advanced (unresectable or metastatic) melanoma who had previously been treated with regimens containing one or more of the following: IL-2, dacarbazine, temozolomide, fotemustine, or carboplatin. Patients were randomized in a 3:1:1 ratio to receive ipilimumab 3 mg/kg + an investigational gp100 peptide vaccine (gp100), ipilimumab 3 mg/kg monotherapy, or gp100 alone. All patients were HLA-A2*0201 type; this HLA type supports the immune presentation of gp100. Patients were enrolled regardless of their baseline BRAF mutation status. Patients received ipilimumab every 3 weeks for 4 doses as tolerated (induction therapy). Patients with apparent tumour burden increase before completion of the induction period were continued on induction therapy as tolerated if they had adequate performance status. Assessment of tumour response to ipilimumab was conducted at approximately Week 12, after completion of
induction therapy.
Additional treatment with ipilimumab (re-treatment) was offered to those who developed PD after initial clinical response (PR or CR) or after SD (per the modified WHO criteria) > 3 months from the first tumour assessment. The primary endpoint was OS in the ipilimumab+ gp100 group vs. the gp100 group. Key secondary endpoints were OS in the ipilimumab+ gp100 group vs. the ipilimumab monotherapy group and in the ipilimumab monotherapy group vs. the gp100 group.
A total of 676 patients were randomized: 137 to the ipilimumab monotherapy group, 403 to the ipilimumab + gp100 group, and 136 to the gp100 alone group. The majority had received all 4 doses during induction. Thirty-two patients received re-treatment: 8 in the ipilimumab monotherapy group, 23 in the ipilimumab + gp100 group, and 1 in the gp100 group. Duration of follow-up ranged up to 55 months. Baseline characteristics were well balanced across groups. The median age was 57 years. The majority (71-73%) of patients had M1c stage disease and 37-40% of patients had an elevated lactate dehydrogenase (LDH) at baseline. A total of 77 patients had a history of previously treated brain metastases.
The ipilimumab-containing regimens demonstrated a statistically significant advantage over the gp100 control group in OS. The hazard ratio (HR) for comparison of OS between ipilimumab monotherapy and gp100 was 0.66 (95% CI: 0.51, 0.87; p = 0.0026).
By subgroup analysis, the observed OS benefit was consistent within most of the subgroups of patients (M [metastases]-stage, prior interleukin-2, baseline LDH, age, sex, and the type and number of prior therapy). However, for women above 50 years of age, the data supporting an OS benefit of ipilimumab treatment were limited. As the subgroups analysis includes only small numbers of patients, no definitive conclusions can be drawn from these data.
Median and estimated rates of OS at 1 year and 2 years are presented in Table 8.
Table 8. Overall survival in MDX010-20:
| Ipilimumab 3 mg/kg n=137 | gp100a n=136 | |
|---|---|---|
| Median Months (95% CI) | 10 months (8.0, 13.8) | 6 months (5.5, 8.7) |
| OS at 1 year % (95% CI) | 46% (37.0, 54.1) | 25% (18.1, 32.9) |
| OS at 2 years % (95% CI) | 24% (16.0, 31.5) | 14% (8.0, 20.0) |
a gp100 peptide vaccine is an experimental control
In the ipilimumab 3 mg/kg monotherapy group, median OS was 22 months and 8 months for patients with SD and those with PD, respectively. At the time of this analysis, medians were not reached for patients with CR or PR.
For patients who required re-treatment, the BORR was 38% (3/8 patients) in the ipilimumab monotherapy group, and 0% in the gp100 group. The disease control rate (DCR) (defined as CR+PR+SD) was 75% (6/8 patients) and 0%, respectively. Because of the limited number of patients in these analyses, no definitive conclusion regarding the efficacy of ipilimumab re-treatment can be drawn.
The development or maintenance of clinical activity following ipilimumab treatment was similar with or without the use of systemic corticosteroids.
CA184-169
A Phase 3, double-blind study enrolled patients with previously treated or untreated unresectable Stage III or Stage IV melanoma. A total of 727 patients were randomized, 362 to receive ipilimumab 3 mg/kg and 365 to receive ipilimumab 10 mg/kg every 3 weeks for 4 doses. In the ipilimumab 10 mg/kg group, the median OS (95% CI) was 16 months (11.63, 17.84) and in the ipilimumab 3 mg/kg group the median OS (95% CI) was 12 months (9.86, 13.27). Overall survival compared between Ipilimumab 10 mg/kg and 3 mg/kg groups showed HR = 0.84 (95% CI: 0.70, 0.99; P-value = 0.04). No statistically significant difference in progression free survival (PFS) was observed between the 10 mg/kg and the 3 mg/kg groups. (HR 0.89 with a 95% CI of 0.76, 1.04 and log-rank test P-value = 0.1548). BORR was similar in the 10 mg/kg and 3 mg/kg groups. BORR in the 10 mg/kg group was 15.3% (95% CI: 11.8, 19.5) and in the 3 mg/kg group was 12.2% (95% CI: 9.0, 16.0). Ipilimumab 10 mg/kg was associated with higher rates of adverse events compared with the 3 mg/kg dose. The frequencies of serious adverse reactions in the 10 mg/kg and 3 mg/kg groups were 37% and 18%, with the 3 most common serious adverse reactions being diarrhea (10.7% vs 5.5%), colitis (8.0% vs 3.0%), and hypophysitis (4.4% vs 1.9%). Adverse events leading to discontinuation in the 10 mg/kg and 3 mg/kg groups occurred in 31% and 19% of patients, with AEs leading to death in 4 and 2 patients, respectively.
At the recommended dose of 3 mg/kg median OS was similar in the subgroup of females ≥50 years of age compared to the overall population (11.40 vs 11.53 months). Median OS in the subgroup with brain metastases at baseline was 5.67 months at the recommended dose of 3 mg/kg.
Other studies with ipilimumab monotherapy
Melanoma
CA184332 and CA184338
OS of ipilimumab 3 mg/kg monotherapy in chemotherapy-naive patients pooled across Phase 2 and 3 clinical trials (N=78; randomised) and in treatment-naive patients in two retrospective observational studies (N=273 and N=157) were generally consistent. In the two observational studies, 12.1% and 33.1% of the patients had brain metastases at the time of advanced melanoma diagnosis. Median OS and estimated 1-year, 2-year, 3-year and 4-year survival rates are presented in Table 9. The estimated 1-year, 2-year and 3-year survival rates for chemotherapy-naive patients (N=78) pooled across Phase 2 and 3 clinical trials were 54.1% (95% CI: 42.5-65.6), 31.6% (95% CI: 20.7-42.9) and 23.7% (95% CI: 14.3-34.4) respectively.
Table 9. Overall survival in observational studies:
| CA184338 n=273 | CA184332 n=157 | |
|---|---|---|
| Median OS (95% CI) | 14 months (12.8-18.7) | 10 months (7.0-12.8) |
| OS at 1 year % (95% CI) | 59% (52.5-64.3) | 44% (35.5, 51.4) |
| OS at 2 years % (95% CI) | 39% (33.1-44.8) | 26% (18.9-33.3) |
| OS at 3 years % (95% CI) | 31% (25.5-36.7) | 22% (15.5-29.2) |
| OS at 4 years % (95% CI) | 26% (20.4-31.3) | 22% (15.5-29.2) |
Patients with brain metastases in study CA184332 had a median OS of 7 months (95% CI: 5.06-12.81) and patients without brain metastases had a median OS of 14.1 months (95% CI: 9.96-Not estimated).
Patients with brain metastases in study CA184338 had a median OS of 6.3 months (95% CI: 3.2-12.0) and patients without brain metastases had a median OS of 17.7 months (95% CI: 13.6–12.1).
Long term survival benefit of treatment with ipilimumab (at 3mg/kg) is demonstrated through a pooled analysis of OS data from clinical trials in patients with previously treated and treatment naive advanced melanoma (N=965). The Kaplan-Meier OS curve revealed a plateau beginning around year 3 (OS rate=21% [95% CI: 17-24]) that extended up to 10 years in some patients (see Figure 1).
Figure 1. Overall Survival with ipilimumab 3 mg/kg in pooled analysis:
Clinical trials with ipilimumab in combination with nivolumab
Melanoma
Randomised phase 3 study of ipilimumab in combination with nivolumab or nivolumab as monotherapy vs. ipilimumab as monotherapy (CA209067)
The safety and efficacy of ipilimumab 3 mg/kg in combination with nivolumab 1 mg/kg nivolumab 3 mg/kg vs. ipilimumab 3 mg/kg monotherapy for the treatment of advanced (unresectable or metastatic) melanoma were evaluated in a phase 3, randomised, double-blind study (CA209067). The differences between the two nivolumab-containing groups were evaluated descriptively. The study included adult patients with confirmed unresectable Stage III or Stage IV melanoma. Patients were to have ECOG performance status score of 0 or 1. Patients who had not received prior systemic anticancer therapy for unresectable or metastatic melanoma were enrolled. Prior adjuvant or neoadjuvant therapy was allowed if it was completed at least 6 weeks prior to randomisation. Patients with active autoimmune disease, ocular/uveal melanoma, or active brain or leptomeningeal metastases were excluded from the study.
A total of 945 patients were randomised to receive ipilimumab in combination with nivolumab (n=314), nivolumab monotherapy (n=316), or ipilimumab monotherapy (n=315). Patients in the combination arm received nivolumab 1 mg/kg over 60 minutes and ipilimumab 3 mg/kg over 90 minutes administered intravenously every 3 weeks for the first 4 doses, followed by nivolumab 3 mg/kg as monotherapy every 2 weeks. Patients in the nivolumab monotherapy arm received nivolumab 3 mg/kg every 2 weeks. Patients in the comparator arm received ipilimumab 3 mg/kg and nivolumab-matched placebo intravenously every 3 weeks for 4 doses followed by placebo every 2 weeks. Randomisation was stratified by PD-L1 expression (≥5% vs. <5% tumour cell membrane expression), BRAF status, and M stage per the American Joint Committee on Cancer (AJCC) staging system. Treatment was continued as long as clinical benefit was observed or until treatment was no longer tolerated. Tumour assessments were conducted 12 weeks after randomisation then every 6 weeks for the first year, and every 12 weeks thereafter. The co-primary outcome measures were progression-free survival and OS. ORR and the duration of response were also assessed.
Baseline characteristics were balanced across the three treatment groups. The median age was 61 years (range: 18 to 90 years), 65% of patients were men, and 97% were white. ECOG performance status score was 0 (73%) or 1 (27%). The majority of the patients had AJCC Stage IV disease (93%); 58% had M1c disease at study entry. Twenty-two percent of patients had received prior adjuvant therapy. Thirty-two percent of patients had BRAF mutation-positive melanoma; 26.5% of patients had PD-L1 ≥5% tumour cell membrane expression. Four percent of patients had a history of brain metastasis, and 36% of patients had a baseline LDH level greater than ULN at study entry. Among patients with quantifiable tumour PD-L1 expression, the distribution of patients was balanced across the three treatment groups. Tumour PD-L1 expression was determined using the PD-L1 IHC 28- 8 pharmDx assay.
At primary analysis (minimum follow-up 9 months) the median PFS was 6.9 months in the nivolumab group as compared with 2.9 months in the ipilimumab group (HR = 0.57, 99.5% CI: 0.43, 0.76; p<0.0001). The median PFS was 11.5 months in the ipilimumab in combination with nivolumab group, as compared with 2.9 months in the ipilimumab group (HR = 0.42, 99.5% CI: 0.31, 0.57; p<0.0001).
PFS results from descriptive analysis (with minimum follow up of 90 months) are shown in Figure 2 (all randomised population), Figure 3 (at the tumour PD-L1 5% cut off), and Figure 4 (at the tumour PD-L1 1% cut off).
Figure 2. Progression-free survival (CA209067):
Figure 3. Progression-free survival by PD-L1 expression: 5% cut off (CA209067):
Figure 4. Progression-free survival by PD-L1 expression: 1% cut off (CA209067):
The final (primary) OS analysis occurred when all patients had a minimum follow-up of 28 months. At 28 months, median OS was not reached in the nivolumab group as compared with 19.98 months in the ipilimumab group (HR = 0.63, 98% CI: 0.48, 0.81; p-value: <0.0001). Median OS was not reached in the ipilimumab in combination with nivolumab group as compared with the ipilimumab group (HR = 0.55, 98% CI: 0.42, 0.72; p-value: <0.0001).
OS results at an additional descriptive analysis undertaken at a minimum follow-up of 90 months show outcomes consistent with the original primary analysis. OS results from this follow-up analysis are shown in Figure 5 (all randomised), Figure 6 and 7 (at the tumour PD-L1 5% and 1% cut off).
The OS analysis was not adjusted to account for subsequent therapies received. Subsequent systemic therapy was received by 36.0%, 49.1%, and 66.3% of patients in the combination, nivolumab monotherapy, and ipilimumab arms, respectively. Subsequent immunotherapy (including anti-PD1 therapy, anti-CTLA-4 antibody, or other immunotherapy) was received by 19.1%, 34.2%, and 48.3% of patients in the combination, nivolumab monotherapy, and ipilimumab arms, respectively.
Figure 5. Overall survival (CA209067) - Minimum follow-up of 36 months:
Figure 6. Overall survival by PD-L1 expression: 5% cut off (CA209067) - Minimum follow-up of 90 months:
Figure 7. Overall survival by PD-L1 expression: 1% cut off (CA209067) - Minimum follow-up of 90 months:
Minimum follow-up for the analysis of ORR was 90 months. Responses are summarised in Table 10.
Table 10. Objective response (CA209067):
| nivolumab + ipilimumab (n=314) | nivolumab (n=316) | ipilimumab (n=315) | |
|---|---|---|---|
| Objective response | 183 (58%) | 142 (45%) | 60 (19%) |
| (95% CI) | (52.6, 63.8) | (39.4, 50.6) | (14.9, 23.8) |
| Odds ratio (vs. ipilimumab) | 6.35 | 3.5 | |
| (99.5% CI) | (4.38, 9.22) | (2.49, 5.16) | |
| Complete response (CR) | 71 (23%) | 59 (19%) | 19 (6%) |
| Partial response (PR) | 112 (36%) | 83 (26%) | 41 (13%) |
| Stable disease (SD) | 38 (12%) | 29 (9%) | 69 (22%) |
| Duration of response | |||
| Median (range), months | N.A. (69.1-N.A.) | 90.8 (45.7-N.A.) | 19.3 (8.8-47.4) |
| Proportion ≥12 months in duration | 68% | 73% | 44% |
| Proportion ≥24 months in duration | 58% | 63% | 30% |
| ORR (95% CI) by tumour PD-L1 expression | |||
| <5% | 56% (48.7, 62.5) n=210 | 43% (36, 49.8) n=208 | 18% (12.8, 23.8) n=202 |
| ≥5% | 72% (59.9, 82.3) n=68 | 59% (47.2, 69.6) n=80 | 21% (12.7, 32.3) n=75 |
| <1% | 54% (44.4, 62.7) n=123 | 36% (27.2, 45.3) n=117 | 18% (11.2, 26.0) n=113 |
| ≥1% | 65% (56.4, 72) n=155 | 55% (47.2, 62.6) n=171 | 20% (13.7, 26.4) n=164 |
Both nivolumab-containing arms demonstrated a significant PFS and OS benefit and greater ORR compared with ipilimumab alone. The observed PFS results at 18 months of follow-up and ORR and OS results at 28 months of follow-up were consistently demonstrated across subgroups of patients including baseline ECOG performance status, BRAF status, M stage, age, history of brain metastases, and baseline LDH level. This observation was maintained with the OS results with a minimum follow-up of 90 months.
Among 131 patients who discontinued the combination due to adverse reaction after 28 months of follow-up, the ORR was 71% (93/131) with 20% (26/131) achieving a complete response and median OS was not reached.
Both nivolumab-containing arms demonstrated greater objective response rates than ipilimumab regardless of PD-L1 expression levels. ORRs were higher for the combination of nivolumab and ipilimumab relative to nivolumab monotherapy across tumour PD-L1 expression levels (Table 10) after 90 months of follow-up, with a best overall response of complete response correlating to an improved survival rate.
After 90 months of follow-up, median durations of response for patients with tumour PD-L1 expression level ≥5% were 78.19 months (range: 18.07-N.A.) in the combination arm, 77.21 months (range: 26.25-N.A.) in the nivolumab monotherapy arm and 31.28 months (range: 6.08-N.A.) in the ipilimumab arm. At tumour PD-L1 expression <5%, median durations of response were not reached (range: 61.93-N.A.) in the combination arm, were 90.84 months (range: 50.43-N.A.) in the nivolumab monotherapy arm and 19.25 months (range: 5.32-47.44) in the ipilimumab monotherapy arm.
No clear cut off for PD-L1 expression can reliably be established when considering the relevant endpoints of tumour response and PFS and OS. Results from exploratory multivariate analyses identified patient and tumour characteristics (ECOG performance status, M stage, baseline LDH, BRAF mutation status, PD-L1 status, and gender) which might contribute to the survival outcome.
Efficacy by BRAF status:
After 90 months of follow-up, BRAF[V600] mutation-positive and BRAF wild-type patients randomised to ipilimumab in combination with nivolumab had a median PFS of 16.76 months(95% CI: 8.28, 32.0) and 11.17 months (95% CI: 7.0, 19.32), while those in the nivolumab monotherapy arm had a median PFS of 5.62 months (95% CI: 2.79, 9.46) and 8.18 months (95% CI: 5.13, 19.55), respectively. BRAF[V600] mutation-positive and BRAF wild-type patients randomised to ipilimumab monotherapy had a median PFS of 3.09 months (95% CI: 2.79, 5.19) and 2.83 months (95% CI: 2.76, 3.06), respectively.
After 90 months of follow-up, BRAF[V600] mutation-positive and BRAF wild-type patients randomised to ipilimumab in combination with nivolumab had an ORR of 67.0% (95% CI: 57.0, 75.9; n=103) and 54.0% (95% CI: 47.1, 60.9; n=211), while those in the nivolumab monotherapy arm had an ORR of 37.87% (95% CI: 28.2, 48.1; n=98) and 48.2% (95% CI: 41.4, 55.0; n=218), respectively. BRAF[V600] mutation-positive and BRAF wild-type patients randomised to ipilimumab monotherapy had an ORR of 23.0% (95% CI: 15.2, 32.5; n=100) and 17.2% (95% CI: 12.4, 22.9; n=215).
After 90 months of follow-up, in BRAF [V600] mutation-positive patients median OS was not reached in the combination arm and 45.5 months in the nivolumab monotherapy arm. Median OS for BRAF [V600] mutation-positive patients in the ipilimumab monotherapy arm was 24.6 months. In BRAF wild-type patients median OS was 39.06 months in the combination arm, 34.37 months in the nivolumab monotherapy arm, and 18.5 months in the ipilimumab monotherapy arm. The OS HRs for ipilimumab in combination with nivolumab vs. nivolumab monotherapy were 0.66 (95% CI: 0.44, 0.98) for BRAF[V600] mutation-positive patients and 0.95 (95% CI: 0.74, 1.22) for BRAF wild-type patients.
Randomised phase 2 study of ipilimumab in combination with nivolumab and ipilimumab (CA209069)
Study CA209069 was a randomised, Phase 2, double-blind study comparing the combination of nivolumab and ipilimumab with ipilimumab alone in 142 patients with advanced (unresectable or metastatic) melanoma with similar inclusion criteria to study CA209067 and the primary analysis in patients with BRAF wild-type melanoma (77% of patients). Investigator assessed ORR was 61% (95% CI: 48.9, 72.4) in the combination arm (n=72) versus 11% (95% CI: 3.0, 25.4) for the ipilimumab arm (n=37). The estimated 2 and 3 year OS rates were 68% (95% CI: 56, 78) and 61% (95% CI: 49, 71), respectively, for the combination (n=73) and 53% (95% CI: 36, 68) and 44% (95% CI: 28, 60), respectively, for ipilimumab (n=37).
Renal Cell Carcinoma (RCC)
Randomised phase 3 study of ipilimumab in combination with nivolumab vs. sunitinib (CA209214)
The safety and efficacy of ipilimumab 1 mg/kg in combination with nivolumab 3 mg/kg for the treatment of advanced/metastatic RCC was evaluated in a phase 3, randomised, open-label study (CA209214). The study included patients (18 years or older) with previously untreated, advanced or metastatic renal cell carcinoma with a clear-cell component. The primary efficacy population included those intermediate/poor risk patients with at least 1 or more of 6 prognostic risk factors as per the International Metastatic RCC Database Consortium (IMDC) criteria (less than one year from time of initial renal cell carcinoma diagnosis to randomisation, Karnofsky performance status <80%, haemoglobin less than the lower limit of normal, corrected calcium of greater than 10 mg/dL, platelet count greater than the upper limit of normal, and absolute neutrophil count greater than the upper limit of normal). This study included patients regardless of their tumour PD-L1 status. Patients with Karnofsky performance status <70% and patients with any history of or concurrent brain metastases, active autoimmune disease, or medical conditions requiring systemic immunosuppression were excluded from the study. Patients were stratified by IMDC prognostic score and region.
A total of 1096 patients were randomised in the trial, of which 847 patients had intermediate/poor-risk RCC and received either ipilimumab 1 mg/kg (n=425) administered intravenously over 30 minutes in combination with nivolumab administered intravenously over 60 minutes every 3 weeks for 4 doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks or sunitinib (n=422) 50 mg daily, administered orally for 4 weeks followed by 2 weeks off, every cycle. Treatment was continued as long as clinical benefit was observed or until treatment was no longer tolerated. The first tumour assessments were conducted 12 weeks after randomisation and continued every 6 weeks thereafter for the first year and then every 12 weeks until progression or treatment discontinuation, whichever occurred later. Treatment beyond initial investigator-assessed RECIST, version 1.1-defined progression was permitted if the patient had a clinical benefit and was tolerating study drug as determined by the investigator. The primary efficacy outcome measures were OS, ORR and PFS as determined by a Blinded Independent Central Review (BICR) in intermediate/poor risk patients.
Baseline characteristics were generally balanced between the two groups. The median age was 61 years (range: 21-85) with 38% ≥65 years of age and 8% ≥75 years of age. The majority of patients were male (73%) and white (87%), and 31% and 69% of patients had a baseline KPS of 70 to 80% and 90 to 100%, respectively. The median duration of time from initial diagnosis to randomisation was 0.4 years in both the ipilimumab 1 mg/kg in combination with nivolumab 3 mg/kg and sunitinib groups. The median duration of treatment was 7.9 months (range: 1 day - 21.4+ months) in ipilimumab with nivolumab-treated patients and was 7.8 months (range: 1 days - 20.2+ months) in sunitinib-treated patients. Ipilimumab with nivolumab was continued beyond progression in 29% of patients.
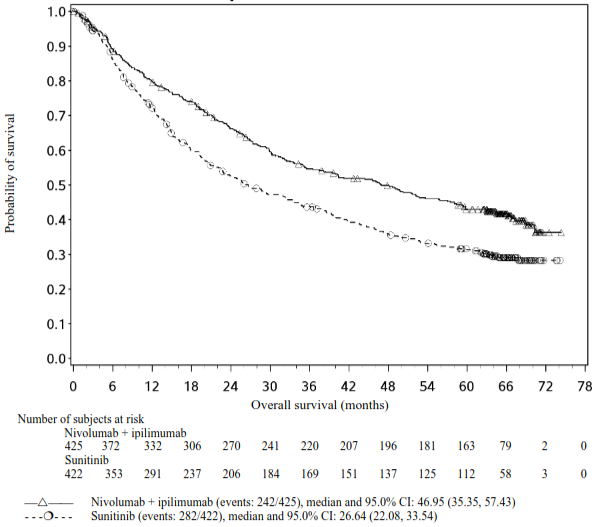
Efficacy results for the intermediate/poor risk patients are shown in Table 11 (primary analysis with a minimum follow-up of 17.5 months and with a minimum follow-up of 60 months) and in Figure 8 (minimum follow-up of 60 months).
OS results at an additional descriptive analysis undertaken at a minimum follow-up of 60 months show outcomes consistent with the original primary analysis.
Table 11. Efficacy results in intermediate/poor risk patients (CA209214):
| nivolumab + ipilimumab (n=425) | sunitinib (n=422) | |
|---|---|---|
| Primary analysis minimum follow-up: 17.5 months | ||
| Overall survival | ||
| Events | 140 (33%) | 188 (45%) |
| Hazard ratioa | 0.63 99.8% CI (0.44, 0.89) | |
| p-valueb,c | <0.0001 | |
| Median (95% CI) | NE (28.2, NE) | 25.9 (22.1, NE) |
| Rate (95% CI) | ||
| At 6 months | 89.5 (86.1, 92.1) | 86.2 (82.4, 89.1) |
| At 12 months | 80.1 (75.9, 83.6) | 72.1 (67.4, 76.2) |
| Progression-free survival | ||
| Events | 228 (53.6%) | 228 (54.0%) |
| Hazard ratioa | 0.82 | |
| 99.1% CI | (0.64, 1.05) | |
| p-valueb,h | 0.0331 | |
| Median (95% CI) | 11.6 (8.71, 15.51) | 8.4 (7.03, 10.81) |
| Confirmed objective response (BICR) | 177 (41.6%) | 112 (26.5%) |
| (95% CI) | (36.9, 46.5) | (22.4, 31.0) |
| Difference in ORR (95% CI)d | 16.0 (9.8, 22.2) | |
| p-valuee,f | <0.0001 | |
| Complete response (CR) | 40 (9.4%) | 5 (1.2%) |
| Partial response (PR) | 137 (32.2%) | 107 (25.4%) |
| Stable disease (SD) | 133 (31.3%) | 188 (44.5%) |
| Median duration of responseg | ||
| Months (range) | NE (1.4±25.5+) | 18.17 (1.3±23.6+) |
| Median time to response | ||
| Months (range) | 2.8 (0.9-11.3) | 3.0 (0.6-15.0) |
| Updated analysis* minimum follow-up: 60 months | ||
| Overall survival | ||
| Events | 242 (57%) | 282 (67%) |
| Hazard ratioa | 0.68 | |
| 95% CI | (0.58, 0.81) | |
| Median (95% CI) | 46.95 (35.35, 57.43) | 26.64 (22.08, 33.54) |
| Rate (95% CI) | ||
| At 24 months | 66.3 (61.5, 70.6) | 52.4 (47.4, 57.1) |
| At 36 months | 54.6 (49.7, 59.3) | 43.7 (38.7, 48.5) |
| At 48 months | 49.9 (44.9, 54.6) | 35.8 (31.1, 40.5) |
| At 60 months | 43.0 (38.1, 47.7) | 31.3 (26.8, 35.9) |
| Progression-free survival | ||
| Events | 245 (57.6%) | 253 (60.0%) |
| Hazard ratioa | 0.73 | |
| 95% CI | (0.61, 0.87) | |
| Median (95% CI) | 11.6 (8.44, 16.63) | 8.3 (7.03, 10.41) |
| Confirmed objective response (BICR) | 179 (42.1%) | 113 (26.8%) |
| (95% CI) | (37.4, 47.0) | (22.6, 31.3) |
| Difference in ORR (95% CI)d,e | 16.2 (10.0, 22.5) | |
| Complete response (CR) | 48 (11.3%) | 9 (2.1%) |
| Partial response (PR) | 131 (30.8%) | 104 (24.6%) |
| Stable disease (SD) | 131 (30.8%) | 187 (44.3%) |
| Median duration of responseg | ||
| Months (range) | NE (50.89-NE) | 19.38 (15.38-25.10) |
| Median time to response | ||
| Months (range) | 2.8 (0.9-35.0) | 3.1 (0.6-23.6) |
a Based on a stratified proportional hazards model.
b Based on a stratified log-rank test.
c p-value is compared to alpha 0.002 in order to achieve statistical significance.
d Strata adjusted difference.
e Based on the stratified DerSimonian-Laird test.
f p-value is compared to alpha 0.001 in order to achieve statistical significance.
g Computed using Kaplan-Meier method.
h p-value is compared to alpha 0.009 in order to achieve statistical significance.
"+" denotes a censored observation.
NE = non-estimable
Figure 8. Kaplan-Meier curves of OS in intermediate/poor risk patients (CA209214) Minimum follow-up of 60 months:
An updated descriptive OS analysis was performed when all patients had a minimum follow-up of 24 months. At the time of this analysis, the hazard ratio was 0.66 (99.8% CI 0.48-0.91) with 166/425 events in the combination arm and 209/422 events in the sunitinib arm. In intermediate/poor-risk patients, OS benefit was observed in the ipilimumab in combination with nivolumab arm vs. sunitinib regardless of tumour PD-L1 expression. Median OS for tumour PD-L1 expression ≥1% was not reached for ipilimumab in combination with nivolumab, and was 19.61 months in the sunitinib arm (HR = 0.52; 95% CI: 0.34, 0.78). For tumour PD-L1 expression <1%, the median OS was 34.7 months for the ipilimumab in combination with nivolumab and was 32.2 months in the sunitinib arm (HR = 0.70; 95% CI: 0.54, 0.92).
CA209214 also randomised 249 favourable risk patients as per IMDC criteria to ipilimumab plus nivolumab (n=125) or to sunitinib (n=124). These patients were not evaluated as part of the primary efficacy population. At a minimum of 24 months follow-up, OS in favourable risk patients receiving ipilimumab plus nivolumab compared to sunitinib had a hazard ratio of 1.13 (95% CI: 0.64, 1.99; p = 0.6710). With 60 months minimum follow-up, the HR for OS was 0.94 (95% CI: 0.65, 1.37).
There are no data on the use of ipilimumab in combination with nivolumab in patients with only a non clear-cell histology in first-line RCC.
Patients ≥75 years of age represented 8% of all intermediate/poor risk patients in CA209214, and the combination of ipilimumab and nivolumab showed numerically less effect on OS (HR 0.97, 95% CI: 0.48, 1.95) in this subgroup versus the overall population at a minimum follow-up of 17.5 months.
Because of the small size of this subgroup, no definitive conclusions can be drawn from these data.
First-line treatment of non-small cell lung cancer
Randomised phase 3 study of ipilimumab in combination with nivolumab and 2 cycles of platinum-based chemotherapy vs. 4 cycles of platinum-based chemotherapy (CA2099LA)
The safety and efficacy of ipilimumab 1 mg/kg every 6 weeks in combination with nivolumab 360 mg every 3 weeks and 2 cycles of platinum-based chemotherapy were evaluated in a phase 3, randomised, open-label study (CA2099LA). The study included patients (18 years or older) with histologically confirmed non-squamous or squamous Stage IV or recurrent NSCLC (per the 7 th International Association for the Study of Lung Cancer classification), ECOG performance status 0 or 1, and no prior anticancer therapy (including EGFR and ALK inhibitors). Patients were enrolled regardless of their tumour PD-L1 status.
Patients with sensitising EGFR mutations or ALK translocations, active (untreated) brain metastases, carcinomatous meningitis, active autoimmune disease, or medical conditions requiring systemic immunosuppression were excluded from the study. Patients with treated brain metastases were eligible if neurologically returned to baseline at least 2 weeks prior to enrolment, and either off corticosteroids, or on a stable or decreasing dose of <10 mg daily prednisone equivalents. Randomisation was stratified by histology (squamous vs non-squamous), tumour PD-L1 expression level (≥1% vs <1%), and gender (male vs female).
A total of 719 patients were randomised to receive either ipilimumab in combination with nivolumab and platinum-based chemotherapy (n=361) or platinum-based chemotherapy (n=358). Patients in the ipilimumab in combination with nivolumab and platinum-based chemotherapy arm received ipilimumab 1 mg/kg administered intravenously over 30 minutes every 6 weeks in combination with nivolumab 360 mg administered intravenously over 30 minutes every 3 weeks and platinum-based chemotherapy administered every 3 weeks for 2 cycles. Patients in the chemotherapy arm received platinum-based chemotherapy administered every 3 weeks for 4 cycles; non-squamous patients could receive optional pemetrexed maintenance therapy.
Platinum-based chemotherapy consisted of carboplatin (AUC 5 or 6) and pemetrexed 500 mg/m²; or cisplatin 75 mg/m² and pemetrexed 500 mg/m² for non-squamous NSCLC; or carboplatin (AUC 6) and paclitaxel 200 mg/m² for squamous NSCLC.
Treatment continued until disease progression, unacceptable toxicity, or for up to 24 months. Treatment could continue beyond disease progression if the patient was clinically stable and was considered to be deriving clinical benefit by the investigator. Patients who discontinued combination therapy because of an adverse event attributed to ipilimumab were permitted to continue nivolumab monotherapy. Tumour assessments were performed every 6 weeks after first dose of study treatment for the first 12 months, then every 12 weeks until disease progression or study treatment was discontinued.
CA2099LA baseline characteristics were generally balanced across all treatment groups. The median age was 65 years (range: 26-86) with 51% ≥65 years of age and 10% ≥75 years of age. The majority of patients were white (89%) and male (70%). Baseline ECOG performance status was 0 (31%) or 1 (68%), 57% of patients with PD-L1 ≥1% and 37% with PD-L1 <1%, 31% had squamous and 69% had non-squamous histology, 17% had brain metastases, and 86% were former/current smokers. No patients received prior immunotherapy.
CA2099LA primary efficacy outcome measure was OS. Additional efficacy endpoints were PFS, ORR, and duration of response as assessed by BICR.
The study demonstrated a statistically significant benefit in OS, PFS, and ORR for patients randomised to ipilimumab in combination with nivolumab and platinum-based chemotherapy as compared to platinum-based chemotherapy alone at the prespecified interim analysis when 351 events were observed (87% of the planned number of events for final analysis). Minimum follow-up for OS was 8.1 months.
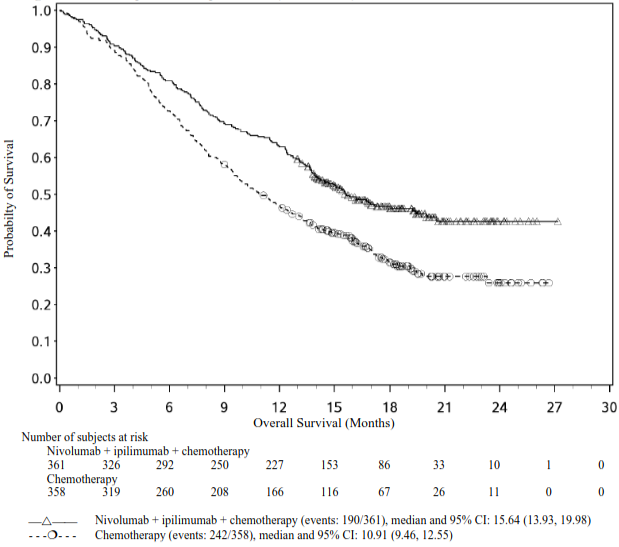
Efficacy results are shown in Figure 9 (updated OS analysis with a minimum follow-up of 12.7 months) and Table 12 (primary analysis with a minimum follow-up of 8.1 months). An updated efficacy analysis was performed when all patients had a minimum follow-up of 12.7 months (see Figure 9). At the time of this analysis, the hazard ratio for OS was 0.66 (95% CI: 0.55, 0.80) and the hazard ratio for PFS was 0.68 (95% CI: 0.57, 0.82).
Figure 9. Kaplan-Meier plot of OS (CA2099LA):
Table 12. Efficacy results (CA2099LA):
| ipilimumab + nivolumab + chemotherapy (n=361) | chemotherapy (n=358) | |
|---|---|---|
| Overall Survival | ||
| Events | 156 (43.2%) | 195 (54.5%) |
| Hazard ratio (96.71% CI)a | 0.69 (0.55, 0.87) | |
| Stratified log-rank p-valueb | 0.0006 | |
| Median (months) (95% CI) | 14.1 (13.24, 16.16) | 10.7 (9.46, 12.45) |
| Rate (95% CI) at 6 months | 80.9 (76.4,84.6) | 72.3 (67.4,76.7) |
| Progression-free Survival | ||
| Events | 232 (64.3%) | 249 (69.6%) |
| Hazard ratio (97.48% CI)a | 0.70 (0.57, 0.86) | |
| Stratified log-rank p-valuec | 0.0001 | |
| Median (months)d (95% CI) | 6.83 (5.55, 7.66) | 4.96 (4.27, 5.55) |
| Rate (95% CI) at 6 months | 51.7 (46.2, 56.8) | 35.9 (30.5, 41.3) |
| Overall Response Ratee | 136 (37.7%) | 90 (25.1%) |
| (95% CI) | (32.7, 42.9) | (20.7, 30.0) |
| Stratified CMH test p-valuef | 0.0003 | |
| Complete response (CR) | 7 (1.9%) | 3 (0.8%) |
| Partial response (PR) | 129 (35.7%) | 87 (24.3%) |
| Duration of Response | ||
| Median (months) (95% CI)d | 10.02 (8.21, 13.01) | 5.09 (4.34, 7.00) |
| % with duration ≥6 monthsg | 74 | 41 |
a Based on a stratified Cox proportional hazard model.
b p-value is compared with the allocated alpha of 0.0329 for this interim analysis.
c p-value is compared with the allocated alpha of 0.0252 for this interim analysis.
d Kaplan-Meier estimate.
e Proportion with complete or partial response; CI based on the Clopper and Pearson Method.
f p-value is compared with the allocated alpha of 0.025 for this interim analysis.
g Based on Kaplan-Meier estimates of duration of response.
CMH = Cochran-Mantel-Haensze
Subsequent systemic therapy was received by 28.8% and 41.1% of patients in the combination and chemotherapy arms, respectively. Subsequent immunotherapy (including anti-PD-1, anti-PD-L1, and anti-CTLA4) was received by 3.9% and 27.9% of patients in the combination and chemotherapy arms, respectively.
In study CA2099LA, subgroup descriptive analysis relative to chemotherapy, OS benefit was shown in patients treated with ipilimumab in combination with nivolumab and chemotherapy with squamous histology (HR [95% CI] 0.65 [0.46, 0.93], n=227) and in patients with non-squamous histology (HR [95% CI] 0.72 [0.55, 0.93], n=492).
Table 13 summarises efficacy results of OS, PFS, and ORR by tumour PD-L1 expression in pre-specified subgroup analyses.
Table 13. Efficacy results by tumour PD-L1 expression (CA2099LA):
| ipilimumab nivolumab chemo- therapy | chemo- therapy | ipilimumab nivolumab chemo- therapy | chemo- therapy | ipilimumab nivolumab chemo- therapy | chemo- therapy | ipilimumab nivolumab chemo- therapy | chemo- therapy | |
|---|---|---|---|---|---|---|---|---|
| PD-L1 <1% (n=264) | PD-L1 ≥1% (n=406) | PD-L1 ≥1% to 49% (n=233) | PD-L1 ≥50% (n=173) | |||||
| OS Hazard Ratio (95% CI)a | 0.65 (0.46, 0.92) | 0.67 (0.51, 0.89) | 0.69 (0.48, 0.98) | 0.64 (0.41, 1.02) | ||||
| PFS Hazard Ratio (95% CI)a | 0.77 (0.57, 1.03) | 0.67 (0.53, 0.85) | 0.71 (0.52, 0.97) | 0.59 (0.40, 0.86) | ||||
| ORR % | 31.1 | 20.9 | 41.9 | 27.6 | 37.8 | 24.5 | 48.7 | 30.9 |
a Hazard ratio based on unstratified Cox proportional hazards model.
A total of 70 NSCLC patients aged ≥75 years were enrolled in study CA2099LA (37 patients in the ipilimumab in combination with nivolumab and chemotherapy arm and 33 patients in the chemotherapy arm). A HR of 1.36 (95% CI: 0.74, 2.52) in OS and a HR of 1.12 (95% CI: 0.64, 1.96) in PFS was observed for ipilimumab in combination with nivolumab and chemotherapy vs. chemotherapy within this study subgroup. ORR was 27.0% in the ipilimumab in combination with nivolumab and chemotherapy arm and 15.2% in the chemotherapy arm. Forty-three percent of patients aged ≥75 years discontinued treatment with ipilimumab in combination with nivolumab and chemotherapy. Efficacy and safety data of ipilimumab in combination with nivolumab and chemotherapy are limited in this patient population.
In a subgroup analysis, a reduced survival benefit for ipilimumab in combination with nivolumab and chemotherapy compared to chemotherapy was observed in patients who were never smokers. However, due to the small numbers of patients, no definitive conclusions can be drawn from these data.
Malignant pleural mesothelioma
Randomised phase 3 study of ipilimumab in combination with nivolumab vs. chemotherapy (CA209743)
The safety and efficacy of ipilimumab 1 mg/kg every 6 weeks in combination with nivolumab 3 mg/kg every 2 weeks were evaluated in a phase 3, randomised, open-label study (CA209743). The study included patients (18 years or older) with histologically confirmed and previously untreated malignant pleural mesothelioma of epithelioid or non-epithelioid histology, ECOG performance status 0 or 1, and no palliative radiotherapy within 14 days of first study therapy. Patients were enrolled regardless of their tumour PD-L1 status.
Patients with primitive peritoneal, pericardial, testis, or tunica vaginalis mesothelioma, interstitial lung disease, active autoimmune disease, medical conditions requiring systemic immunosuppression, and brain metastasis (unless surgically resected or treated with stereotaxic radiotherapy and no evolution within 3 months prior to inclusion in the study) were excluded from the trial. Randomisation was stratified by histology (epithelioid vs. sarcomatoid or mixed histology subtypes) and gender (male vs. female).
A total of 605 patients were randomised to receive either ipilimumab in combination with nivolumab (n=303) or chemotherapy (n=302). Patients in the ipilimumab in combination with nivolumab arm received ipilimumab 1 mg/kg over 30 minutes by intravenous infusion every 6 weeks in combination with nivolumab 3 mg/kg over 30 minutes by intravenous infusion every 2 weeks for up to 2 years. Patients in the chemotherapy arm received chemotherapy for up to 6 cycles (each cycle was 21 days). Chemotherapy consisted of cisplatin 75 mg/m² and pemetrexed 500 mg/m² or carboplatin 5 AUC and pemetrexed 500 mg/m².
Treatment continued until disease progression, unacceptable toxicity, or for up to 24 months. Treatment could continue beyond disease progression if the patient was clinically stable and was considered to be deriving clinical benefit by the investigator. Patients who discontinued combination therapy because of an adverse reaction attributed to ipilimumab were permitted to continue nivolumab monotherapy. Tumour assessments were performed every 6 weeks after first dose of study treatment for the first 12 months, then every 12 weeks until disease progression or study treatment was discontinued.
CA209743 baseline characteristics were generally balanced across all treatment groups. The median age was 69 years (range: 25-89) with 72% ≥65 years of age and 26% ≥75 years of age. The majority of patients were white (85%) and male (77%). Baseline ECOG performance status was 0 (40%) or 1 (60%), 80% of patients with PD-L1 ≥1% and 20% with PD-L1 <1%, 75% had epithelioid and 25% had non-epithelioid histology.
CA209743 primary efficacy outcome measure was OS. Key secondary efficacy endpoints were PFS, ORR, and duration of response as assessed by BICR utilising modified RECIST criteria for pleural mesothelioma. Descriptive analyses for these secondary endpoints are presented in Table 14.
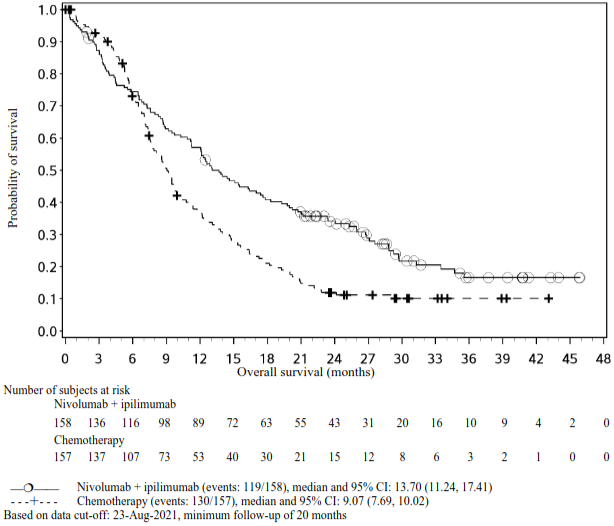
The study demonstrated a statistically significant improvement in OS for patients randomised to ipilimumab in combination with nivolumab as compared to chemotherapy at the prespecified interim analysis when 419 events were observed (89% of the planned number of events for final analysis). Minimum follow-up for OS was 22 months.
Efficacy results are shown in Figure 10 and Table 14.
Figure 10. Kaplan-Meier curves of OS (CA209743):
Table 14. Efficacy results (CA209743):
| ipilimumab + nivolumab (n=303) | chemotherapy (n=302) | |
|---|---|---|
| Overall survival | ||
| Events | 200 (66%) | 219 (73%) |
| Hazard ratio (96.6% CI)a | 0.74 (0.60, 0.91) | |
| Stratified log-rank p-valueb | 0.002 | |
| Median (months)c (95% CI) | 18.1 (16.8, 21.5) | 14.1 (12.5, 16.2) |
| Rate (95% CI) at 24 monthsc | 41% (35.1, 46.5) | 27% (21.9, 32.4) |
| Progression-free survival | ||
| Events | 218 (72%) | 209 (69%) |
| Hazard ratio (95% CI)a | 1.0 (0.82, 1.21) | |
| Median (months)c (95% CI) | 6.8 (5.6, 7.4) | 7.2 (6.9, 8.1) |
| Overall response rate | 40% | 43% |
| (95% CI) | (34.1, 45.4) | (37.1, 48.5) |
| Complete response (CR) | 1.7% | 0 |
| Partial response (PR) | 38% | 43% |
| Duration of response | ||
| Median (months)c (95% CI) | 11.0 (8.1, 16.5) | 6.7 (5.3, 7.1) |
a Stratified Cox proportional hazard model.
b p-value is compared with the allocated alpha of 0.0345 for this interim analysis.
c Kaplan-Meier estimate.
Subsequent systemic therapy was received by 44.2% and 40.7% of patients in the combination and chemotherapy arms, respectively. Subsequent immunotherapy (including anti-PD-1, anti-PD-L1, and anti-CTLA-4) was received by 3.3% and 20.2% of patients in the combination and chemotherapy arms, respectively.
Table 15 summarises efficacy results of OS, PFS, and ORR by histology in prespecified subgroup analyses.
Table 15. Efficacy results by histology (CA209743):
| Epithelioid (n=471) | Non-epithelioid (n=134) | |||
|---|---|---|---|---|
| ipilimumab + nivolumab (n=236) | chemotherapy (n=235) | ipilimumab + nivolumab (n=67) | chemotherapy (n=67) | |
| Overall survival | ||||
| Events | 157 | 164 | 43 | 55 |
| Hazard ratio (95% CI)a | 0.85 (0.68, 1.06) | 0.46 (0.31, 0.70) | ||
| Median (months) (95% CI) | 18.73 (17.05, 21.72) | 16.23 (14.09, 19.15) | 16.89 (11.83, 25.20) | 8.80 (7.62, 11.76) |
| Rate (95% CI) at 24 months | 41.2 (34.7, 47.6) | 31.8 (25.7, 38.1) | 39.5 (27.5, 51.2) | 9.7 (3.8, 18.9) |
| Progression-free survival | ||||
| Hazard ratio (95% CI)a | 1.14 (0.92, 1.41) | 0.58 (0.38, 0.90) | ||
| Median (months) (95% CI) | 6.18 (5.49, 7.03) | 7.66 (7.03, 8.31) | 8.31 (3.84, 11.01) | 5.59 (5.13, 7.16) |
| Overall response rate | 38.6% | 47.2% | 43.3% | 26.9% |
| (95% CI)b | (32.3, 45.1) | (40.7, 53.8) | (31.2, 56.0) | (16.8, 39.1) |
| Duration of response | 8.44 | 6.83 | 24.02 | 4.21 |
| Median (months) (95% CI)c | (7.16, 14.59) | (5.59, 7.13) | (8.31, N.A.) | (2.79, 7.03) |
a Hazard ratio based on unstratified Cox proportional hazards model.
b Confidence interval based on the Clopper and Pearson method.
c Median computed using Kaplan-Meier method.
Table 16 summarises efficacy results of OS, PFS, and ORR by baseline tumour PD-L1 expression in prespecified subgroup analyses.
Table 16. Efficacy results by tumour PD-L1 expression (CA209743):
| PD-L1 <1% (n=135) | PD-L1 ≥1% (n=451) | |||
|---|---|---|---|---|
| ipilimumab + nivolumab (n=57) | chemotherapy (n=78) | ipilimumab + nivolumab (n=232) | chemotherapy (n=219) | |
| Overall survival | ||||
| Events | 40 | 58 | 150 | 157 |
| Hazard ratio (95% CI)a | 0.94 (0.62, 1.40) | 0.69 (0.55, 0.87) | ||
| Median (months) (95% CI)b | 17.3 (10.1, 24.3) | 16.5 (13.4, 20.5) | 18.0 (16.8, 21.5) | 13.3 (11.6, 15.4) |
| Rate (95% CI) at 24 months | 38.7 (25.9, 51.3) | 24.6 (15.5, 35.0) | 40.8 (34.3, 47.2) | 28.3 (22.1, 34.7) |
| Progression-free survival | ||||
| Hazard ratio (95% CI)a | 1.79 (1.21, 2.64) | 0.81 (0.64, 1.01) | ||
| Median (months) (95% CI)b | 4.1 (2.7, 5.6) | 8.3 (7.0, 11.1) | 7.0 (5.8, 8.5) | 7.1 (6.2, 7.6) |
| Overall response rate | 21.1% | 38.5% | 43.5% | 44.3% |
| (95% CI)c | (11.4, 33.9) | (27.7, 50.2) | (37.1, 50.2) | (37.6, 51.1) |
a Hazard ratio based on unstratified Cox proportional hazards model.
b Median computed using Kaplan-Meier method.
c Confidence interval based on the Clopper and Pearson method.
A total of 157 MPM patients aged ≥75 years were enrolled in study CA209743 (78 in the ipilimumab in combination with nivolumab arm and 79 in the chemotherapy arm). A HR of 1.02 (95% CI: 0.70, 1.48) in OS was observed for ipilimumab in combination with nivolumab vs. chemotherapy within this study subgroup. A higher rate of serious adverse reactions and discontinuation rate due to adverse reactions in patients 75 years of age or older relative to all patients who received ipilimumab in combination with nivolumab was shown (see section 4.8). However, due to the exploratory nature of this subgroup analysis, no definitive conclusions can be drawn.
dMMR or MSI-H colorectal cancer
The safety and efficacy of ipilimumab 1 mg/kg in combination with nivolumab 3 mg/kg for the treatment of dMMR or MSI-H metastatic CRC was evaluated in a Phase 2, multi-centre, open-label, single-arm study (CA209142).
The study included patients (18 years or older) with locally determined dMMR or MSI-H status, who had disease progression during, after, or were intolerant to, prior therapy with fluoropyrimidine and oxaliplatin or irinotecan. Patients who had their most recent prior treatment in the adjuvant setting should have progressed on or within 6 months of completion of adjuvant chemotherapy. Patients had an ECOG performance status score of 0 or 1 and were enrolled regardless of their tumour PD-L1 status. Patients with active brain metastases, active autoimmune disease, or medical conditions requiring systemic immunosuppression were excluded from the study.
A total of 119 patients were treated with ipilimumab 1 mg/kg administered intravenously over 90 minutes in combination with nivolumab 3 mg/kg administered intravenously over 60 minutes every 3 weeks for 4 doses followed by nivolumab monotherapy 3 mg/kg every 2 weeks. Treatment was continued as long as clinical benefit was observed or until treatment was no longer tolerated. Tumour assessments according to RECIST version 1.1 were conducted every 6 weeks for the first 24 weeks and every 12 weeks thereafter. The primary outcome measure was investigator-assessed ORR. Secondary outcome measures were BICR-assessed ORR and disease control rate. Analysis of ORR included duration of and time to response. Exploratory outcome measures included PFS and OS.
The median age was 58 years (range: 21-88) with 32% ≥65 years of age and 9% ≥75 years of age, 59% were male and 92% were white. Baseline ECOG performance status was 0 (45%) or 1 (55%), 25% of patients had BRAF mutations, 37% had KRAS mutations, and 12% were unknown. Of the 119 treated patients, 109 had received prior fluoropyrimidine based chemotherapy in the metastatic setting and 9 in the adjuvant setting. Before study enrolment, of the 119 treated patients, 118 (99%) had received fluorouracil, 111 (93%) had received oxaliplatin, 87 (73%) had received irinotecan as part of prior therapies; 82 (69%) had received prior treatment with fluoropyrimidine, oxaliplatin, and irinotecan. Twenty three percent, 36%, 24%, and 16% received 1, 2, 3, or 4 or more prior therapies respectively, and 29% of patients had received an EGFR inhibitor.
Efficacy results (minimum follow-up 46.9 months; median follow-up 51.1 months) are shown in Table 17.
Table 17. Efficacy results (CA209142) in dMMR or MSI-H CRC patients*:
| ipilimumab + nivolumab (n=119) | |
|---|---|
| Confirmed objective response, n (%) | 77 (64.7) |
| (95% CI) | (55.4, 73.2) |
| Complete response (CR), n (%) | 15 (12.6) |
| Partial response (PR), n (%) | 62 (52.1) |
| Stable disease (SD), n (%) | 25 (21.0) |
| Duration of response | |
| Median (range) months | NR (1.4, 58.0+) |
| Median time to response | |
| Months (range) | 2.8 (1.1, 37.1) |
* per investigator assessment
" +" denotes a censored observation.
NR = not reached
The BICR-assessed ORR was 61.3% (95% CI: 52.0, 70.1), including CR rate of 20.2% (95% CI: 13.4, 28.5), PR rate of 41.2% (95% CI: 32.2, 50.6) and stable disease reported in 22.7%. BICR assessments were generally consistent with the investigator assessment. Confirmed responses were observed regardless of BRAF or KRAS mutation status, and tumour PD-L1 expression levels.
Of 119 patients 11 (9.2%) patients were ≥75 years. The investigator assessed ORR in patients ≥75 years was 45.5% (95% CI: 16.7, 76.6).
Oesophageal squamous cell carcinoma
Randomised phase 3 study of ipilimumab in combination with nivolumab vs. chemotherapy as first-line treatment (CA209648)
The safety and efficacy of ipilimumab in combination with nivolumab were evaluated in a randomised, active-controlled, open-label phase 3 study (CA209648). The study included adult patients (18 years or older) with previously untreated, unresectable advanced, recurrent or metastatic oesophageal squamous cell carcinoma (OSCC). Patients were enrolled regardless of their tumour PD-L1 status, and tumour cell PD-L1 expression was determined using the PD-L1 IHC 28-8 pharmDx assay. Patients were required to have squamous cell carcinoma or adenosquamous cell carcinoma of oesophagus, not amenable to chemoradiation and/or surgery. Prior adjuvant, neoadjuvant, or definitive, chemotherapy, radiotherapy or chemoradiotherapy was permitted if given as part of curative intent regimen prior to trial enrollment. Patients who had a baseline performance score ≥2, had brain metastases that were symptomatic, had active autoimmune disease, used systemic corticosteroids or immunosuppressants, or patients at high risk of bleeding or fistula due to apparent invasion of tumour to organs adjacent to the oesophageal tumour were excluded from the study. Randomisation was stratified by tumour cell PD-L1 status (≥1% vs. < 1% or indeterminate), region (East Asia vs. rest of Asia vs. rest of world), ECOG performance status (0 vs. 1), and number of organs with metastases (≤1 vs. ≥2).
A total of 649 patients were randomised to receive either ipilimumab in combination with nivolumab (n=325), or chemotherapy (n=324), respectively. Of these, 315 patients had tumour cell PD-L1 expression ≥1%, 158 in the ipilimumab plus nivolumab arm and 157 in the chemotherapy arm. Patients in the ipilimumab plus nivolumab arm received ipilimumab 1 mg/kg every 6 weeks in combination with nivolumab 3 mg/kg every 2 weeks. Patients in the chemotherapy arm received fluorouracil 800 mg/m²/day intravenously on days 1 through 5 (for 5 days), and cisplatin 80 mg/m² intravenously on day 1 (of a 4-week cycle). Treatment continued until disease progression, unacceptable toxicity, or up to 24 months. Patients who discontinued combination therapy because of an adverse reaction attributed to ipilimumab were permitted to continue nivolumab as a single agent.
Baseline characteristics were generally balanced across treatment groups. In patients with tumour cell PD-L1 expression ≥1%, the median age was 63 years (range: 26-85), 8.2% were ≥75 years of age, 81.8% were male, 73.1% were Asian, and 23.3% were white. Patients had histological confirmation of squamous cell carcinoma (98.9%) or adenosquamous cell carcinoma (1.1%) in the oesophagus. Baseline ECOG performance status was 0 (45.2%) or 1 (54.8%).
The primary efficacy outcome measures were PFS (by BICR) and OS assessed in patients with tumour cell PD-L1expression ≥1%. Secondary endpoints per the pre-specified hierarchical testing incuded OS, PFS (by BICR), and ORR (by BICR) in all randomised patients. The tumour assessments per RECIST v1.1 were conducted every 6 weeks up to and including week 48, then every 12 weeks thereafter.
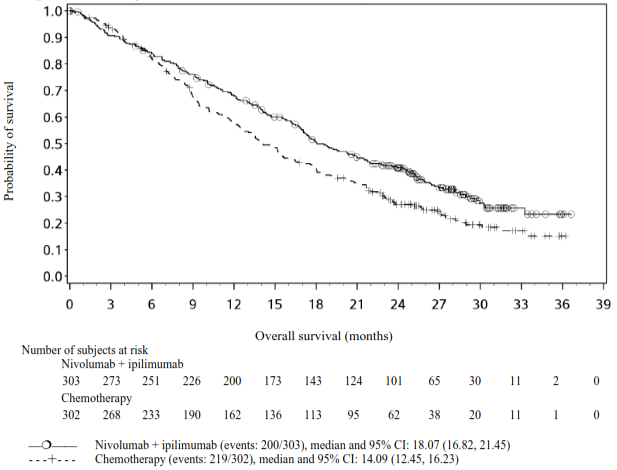
At the primary pre-specified analysis, with a minimum follow-up of 13.1 months, the study demonstrated a statistically significant improvement in OS in patients with tumour cell PD-L1 expression ≥1%. Efficacy results are shown in Table 18.
Table 18. Efficacy results in patients with tumour cell PD-L1 ≥1% (CA209648):
| ipilimumab + nivolumab (n=158) | chemotherapya (n=157) | |
|---|---|---|
| Overall survival | ||
| Events | 106 (67.1%) | 121 (77.1%) |
| Hazard ratio (98.6% CI)b | 0.64 (0.46, 0.90) | |
| p-valuec | 0.0010 | |
| Median (95% CI) (months)d | 13.70 (11.24, 17.02) | 9.07 (7.69, 9.95) |
| Rate (95% CI) at 12 monthsd | 57.1 (49.0, 64.4) | 37.1 (29.2, 44.9) |
| Progression-free survivale | ||
| Events | 123 (77.8%) | 100 (63.7%) |
| Hazard ratio (98.5% CI)b | 1.02 (0.73, 1.43) | |
| p-valuec | 0.8958 | |
| Median (95% CI) (months)d | 4.04 (2.40, 4.93) | 4.44 (2.89, 5.82) |
| Rate (95% CI) at 12 monthsd | 26.4 (19.5, 33.9) | 10.5 (4.7, 18.8) |
| Overall response rate, n (%)e | 56 (35.4) | 31 (19.7) |
| (95% CI) | (28.0, 43.4) | (13.8, 26.8) |
| Complete response | 28 (17.7) | 8 (5.1) |
| Partial response | 28 (17.7) | 23 (14.6) |
| Duration of responsee | ||
| Median (95% CI) (months)d | 11.83 (7.10, 27.43) | 5.68 (4.40, 8.67) |
| Range | 1.4+, 34.5+ | 1.4+, 31.8+ |
a Fluorouracil and cisplatin.
b Based on stratified Cox proportional hazard model.
c Based on stratified 2-sided log-rank test.
d Based on Kaplan-Meier estimates.
e Assessed by BICR.
At an updated descriptive analysis with a minimum follow-up of 20 months, OS improvements were consistent with the primary analysis. Median OS was 13.70 months (95% CI: 11.24, 17.41) for ipilimumab plus nivolumab vs. 9.07 months (95% CI: 7.69, 10.02) for chemotherapy (HR = 0.63; 95% CI: 0.49, 0.82). Median PFS was 4.04 months (95% CI: 2.40, 4.93) for ipilimumab plus nivolumab vs. 4.44 months (95% CI: 2.89, 5.82) for chemotherapy (HR = 1.02; 95% CI: 0.77, 1.34). The ORR was 35.4% (95% CI: 28.0, 43.4) for ipilimumab plus nivolumab vs. 19.7% (95% CI: 13.8, 26.8) for chemotherapy.
The Kaplan-Meier curves for OS with a minimum follow-up of 20 months are shown in Figure 11.
Figure 11. Kaplan-Meier curves of OS in patients with tumour cell PD-L1 ≥1% (CA209648):
Paediatric population
Ipilimumab as monotherapy
Study CA184070 was a multi-center, Phase 1, open-label, dose-escalation study of ipilimumab in paediatric patients ≥1 year to ≤21 years of age with measurable/evaluable, untreatable, relapsed or refractory solid malignant tumours without a curative option with standard therapy. The study enrolled 13 patients <12 of age and 20 patients ≥12 years of age. Ipilimumab was administered every 3 weeks for 4 doses and then every 12 weeks thereafter in the absence of dose limiting toxicity (DLT) and disease progression. The primary endpoints were safety and pharmacokinetics (PK). Of patients 12 years of age and older with advanced melanoma, ipilimumab 5 mg/kg was administered to three patients and ipilimumab 10 mg/kg was administered to two patients. Stable disease was achieved in two patients at the ipilimumab 5 mg/kg dose, one with a duration of >22 months.
Study CA184178 was a non-randomized, multicenter, open-label Phase 2 study, in adolescent patients 12 to <18 years of age with previously treated or untreated, unresectable Stage III or Stage IV malignant melanoma. Ipilimumab was administered every 3 weeks for 4 doses. The primary efficacy endpoint was 1-year survival rate. Secondary efficacy endpoints of best overall response rate (BORR), stable disease (SD), disease control rate (DCR), and progression free survival (PFS) were based on mWHO criteria and determined by the investigator's assessment. Overall survival (OS) was also evaluated. Tumour assessment was performed at Week 12. All patients were followed for at least 1 year. Ipilimumab 3 mg/kg was administered to four patients and ipilimumab 10 mg/kg was administered to eight patients. Most patients were male (58%) and white (92%). Median age was 15 years. Stable disease was achieved for 260 days in one patient on ipilimumab 3 mg/kg and approximately 14 months in one patient on ipilimumab 10 mg/kg. Two patients treated with 59 ipilimumab 10 mg/kg experienced a partial response, one of which was a durable response for more than 1 year. Additional efficacy results are presented in Table 19.
Table 19. Efficacy Results in CA184178:
| Ipilimumab 3 mg/kg N=4 | Ipilimumab 10 mg/kg N=8 | |
|---|---|---|
| 1-year OS (%) (95% CI) | 75% (12.8, 96.1) | 62.5% (22.9, 86.1) |
| BORR (%) (95% CI) | 0% (0, 60.2) | 25% (3.2, 65.1) |
| SD (n/N)a | ¼ | 1/8 |
| DCR (%) (95% CI) | 25% (0.6, 80.6) | 37.5% (8.5, 75.5) |
| Median PFS (months) (95% CI) | 2.6 (2.3, 8.5) | 2.9 (0.7, NEa) |
| Median OS (months) (95% CI) | 18.2 (8.9, 18.2) | Not reached (5.2, NE) |
a NE= not estimable
Ipilimumab in combination with nivolumab
Study CA209070 was an open-label, single-arm, dose-confirmation and dose-expansion, phase ½ study of nivolumab as a single agent and in combination with ipilimumab in paediatric and young adult patients with recurrent or refractory solid or haematological tumours, including neuroblastoma, osteosarcoma, rhabdomyosarcoma, Ewing sarcoma, advanced melanoma, cHL and non-Hodgkin lymphoma (NHL). Among the 126 treated patients, 97 were paediatric patients from 12 months to <18 years of age. Of the 97 paediatric patients, 64 were treated with nivolumab monotherapy (3 mg/kg administered intravenously over 60 minutes every 2 weeks) and 33 were treated with ipilimumab in combination with nivolumab (nivolumab 1 mg/kg or 3 mg/kg administered intravenously over 60 minutes in combination with ipilimumab 1 mg/kg administered intravenously over 90 minutes every 3 weeks for the first 4 doses, followed by nivolumab 3 mg/kg as monotherapy every 2 weeks). Patients received either nivolumab as monotherapy for a median of 2 doses (range: 1, 89) or ipilimumab in combination with nivolumab for a median of 2 doses (range: 1, 24). The main primary outcome measures were safety, tolerability and antitumour activity as evaluated by descriptive ORR and OS.
Among the 64 paediatric patients treated with nivolumab monotherapy, 60 were response-evaluable patients (melanoma n=1, solid tumours n=47 and haematological tumours n=12). In the 48 response-evaluable paediatric patients with melanoma or solid tumours, no objective responses were observed. In the 12 response-evaluable paediatric patients with haematological tumours, ORR was 25.0% (95% CI: 5.5, 57.2), including 1 complete response in cHL and 2 partial responses, one in cHL and another one in NHL. In the descriptive analyses for the 64 paediatric patients treated with nivolumab monotherapy, the median OS was 6.67 months (95% CI: 5.98, NA); 6.14 months (95% CI: 5.39, 24.67) for patients with melanoma or solid tumours, and not reached for patients with haematological tumours.
Among the 30 response-evaluable paediatric patients treated with ipilimumab in combination with nivolumab (solid tumours other than melanoma only), no objective responses were observed. For the 33 paediatric patients treated with ipilimumab in combination with nivolumab, the median OS was 8.25 months (95% CI: 5.45, 16.95) in a descriptive analyses.
Study CA209908 was an open-label, sequential-arm, phase 1b/2 clinical study of nivolumab monotherapy and ipilimumab in combination with nivolumab in paediatric and young adult patients with high-grade primary CNS malignancies, including diffuse intrinsic pontine glioma (DIPG), high-grade glioma, medulloblastoma, ependymoma and other recurrent subtypes of high-grade CNS malignancy (e.g., pineoblastoma, atypical teratoid/rhabdoid tumour, and embryonal CNS tumours). Of the 151 paediatric patients (from ≥6 months to <18 years old) enrolled in the study, 77 were treated with nivolumab monotherapy (3 mg/kg every 2 weeks) and 74 were treated with ipilimumab 1 mg/kg in combination with nivolumab 3 mg/kg, every 3 weeks for 4 doses, followed by nivolumab monotherapy 3 mg/kg every 2 weeks. The primary efficacy outcome measures were OS in the DIPG cohort and investigator-assessed PFS, based on RANO criteria, for all other tumour types. The median OS in the DIPG cohort was 10.97 months (80% CI: 9.92, 12.16) in patients treated with nivolumab monotherapy and 10.50 months (80% CI: 9.10, 12.32) in patients treated with ipilimumab in combination with nivolumab. For all other studied CNS paediatric tumour types, the median PFS ranged from 1.23 to 2.35 months in patients treated with nivolumab monotherapy and from 1.45 to 3.09 months in patients treated with ipilimumab in combination with nivolumab. There were no objective responses observed in the study with the exception of one ependymoma patient treated with nivolumab monotherapy who had a partial response. Results for OS, PFS, and ORR observed in study CA209908 do not suggest clinically meaningful improvement over what is expected in these patient populations.
Pharmacokinetic properties
The pharmacokinetics of ipilimumab was studied in 785 patients with advanced melanoma who received induction doses ranging from 0.3 to 10 mg/kg administered once every 3 weeks for 4 doses. Cmax, Cmin and AUC of ipilimumab were found to be dose proportional within the dose range examined. Upon repeated dosing of ipilimumab administered every 3 weeks, CL was found to be time-invariant, and minimal systemic accumulation was observed as evident by an accumulation index 1.5 fold or less. Ipilimumab steady-state was reached by the third dose. Based on population pharmacokinetic analysis, the following mean (percent coefficient of variation) parameters of ipilimumab were obtained: terminal half-life of 15.4 days (34.4%); systemic CL of 16.8 ml/h (38.1%); and volume of distribution at steady-state of 7.47 l (10.1%). The mean (percent coefficient of variation) ipilimumab Cmin achieved at steady-state with a 3 mg/kg induction regimen was 19.4 μg/ml (74.6%).
Ipilimumab CL increased with increasing body weight and with increasing LDH at baseline; however, no dose adjustment is required for elevated LDH or body weight after administration on a mg/kg basis. CL was not affected by age (range 23-88 years), gender, concomitant use of budesonide or dacarbazine, performance status, HLA-A2*0201 status, mild hepatic impairment, renal impairment, immunogenicity, and previous anticancer therapy. The effect of race was not examined as there was insufficient data in non-Caucasian ethnic groups. No controlled studies have been conducted to evaluate the pharmacokinetics of ipilimumab in the paediatric population or in patients with hepatic or renal impairment.
Based on an exposure-response analysis in 497 patients with advanced melanoma, OS was independent of prior systemic anti-cancer therapy and increased with higher ipilimumab Cminss plasma concentrations.
Yervoy in combination with nivolumab: When ipilimumab 1 mg/kg was administered in combination with nivolumab 3 mg/kg, the CL of ipilimumab was decreased by 1.5% and the CL of nivolumab was increased by 1% which were not considered clinically relevant. When ipilimumab 3 mg/kg was administered in combination with nivolumab 1 mg/kg, the CL of ipilimumab was increased by 9% and the CL of nivolumab was increased by 29%, which was not considered clinically relevant.
When administered in combination with nivolumab, the CL of ipilimumab increased by 5.7% in the presence of anti-ipilimumab antibodies and the CL of nivolumab increased by 20% in the presence of presence of anti-nivolumab antibodies. These changes were not considered clinically relevant.
Yervoy in combination with nivolumab and chemotherapy: When ipilimumab 1 mg/kg every 6 weeks was administered in combination with nivolumab 360 mg every 3 weeks and with 2 cycles of chemotherapy, the CL of ipilimumab increased approximately 22% and the CL of nivolumab decreased approximately 10%, which was not considered clinically relevant.
Renal impairment
In the population pharmacokinetic analysis of data from clinical studies in patients with metastatic melanoma, pre-existing mild and moderate renal impairment did not influence the CL of ipilimumab. Clinical and pharmacokinetic data with pre-existing severe renal impairment are limited; the potential need for dose adjustment cannot be determined.
Hepatic impairment
In the population pharmacokinetic analysis of data from clinical studies in patients with metastatic melanoma, pre-existing mild hepatic impairment did not influence the CL of ipilimumab. Clinical and pharmacokinetic data with pre-existing moderate hepatic impairment are limited; the potential need for dose adjustment cannot be determined. No patients with pre-existing severe hepatic impairment were identified in clinical studies.
Paediatric population
For ipilimumab monotherapy, based on a population PK analysis using available pooled data from 565 patients from 4 phase 2 adult studies (N=521) and 2 paediatric studies (N=44), CL of ipilimumab increased with increasing baseline body weight. Age (2-87 years) had no clinically important effect on the CL of ipilimumab. The estimated geometric mean CL is 8.72 ml/h in adolescent patients aged ≥12 to <18 years. Exposures in adolescents are comparable with those in adults receiving the same mg/kg dose. Based on the simulation in adults and paediatrics, comparable exposure is achieved in adults and paediatrics at the recommended dose of 3 mg/kg every 3 weeks.
For ipilimumab in combination with nivolumab, the exposures of ipilimumab and nivolumab in pediatric patients 12 years of age and older are expected to be comparable to that in adult patients at the recommended dose.
Preclinical safety data
In intravenous repeat-dose toxicology studies in monkeys, ipilimumab was generally well tolerated. Immune-mediated adverse reactions were observed infrequently (~3%) and included colitis (which resulted in a single fatality), dermatitis, and infusion reaction (possibly due to acute cytokine release resulting from a rapid injection rate). A decrease in the weight of the thyroid and testes was seen in one study without accompanying histopathologic findings; the clinical relevance of this finding is unknown.
The effects of ipilimumab on prenatal and postnatal development were investigated in a study in cynomolgus monkeys. Pregnant monkeys received ipilimumab every 3 weeks from the onset of organogenesis in the first trimester through delivery, at exposure (AUC) levels either similar to or higher than those associated with the clinical dose of 3 mg/kg of ipilimumab. No treatment-related adverse effects on reproduction were detected during the first two trimesters of pregnancy. Beginning in the third trimester, both ipilimumab groups experienced higher incidences of abortion, stillbirth, premature delivery (with corresponding lower birth weight), and infant mortality relative to control animals; these findings were dose-dependent. Additionally, developmental external or visceral abnormalities were identified in the urogenital system of 2 infants exposed in utero to ipilimumab. One female infant had unilateral renal agenesis of the left kidney and ureter, and one male infant had an imperforate urethra with associated urinary obstruction and subcutaneous scrotal edema. The relationship of these malformations to treatment is unclear.
Studies to evaluate the mutagenic and carcinogenic potential of ipilimumab have not been performed. Fertility studies have not been performed.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.