ZAVZPRET Spray Ref.[107356] Active ingredients: Zavegepant
Source: FDA, National Drug Code (US) Revision Year: 2023
12.1. Mechanism of Action
Zavegepant is a calcitonin gene-related peptide (CGRP) receptor antagonist.
12.2. Pharmacodynamics
The relationship between pharmacodynamic activity and the mechanism by which zavegepant exerts its clinical effects is unknown.
No clinically relevant differences in resting blood pressure were observed when zavegepant was concomitantly administered with sumatriptan (12 mg subcutaneous, given as two 6 mg doses separated by one hour) compared with sumatriptan alone to healthy volunteers.
Cardiac Electrophysiology
At a dose up to 4 times the recommended daily dose, zavegepant does not prolong the QT interval to any clinically relevant extent.
12.3. Pharmacokinetics
Absorption
Peak plasma concentration of zavegepant was observed at approximately 30 minutes after a single 10 mg dose of the nasal spray. After nasal spray administration of zavegepant, the absolute bioavailability is approximately 5%.
Zavegepant given as a single dose of the nasal spray displays slightly less than dose-proportional pharmacokinetics up to 40 mg (approximately 4 times the recommended dosage of 10 mg).
Following once daily dosing of ZAVZPRET for 14 days there was no evidence of zavegepant accumulation.
Distribution
The mean apparent volume of distribution of intranasal zavegepant is approximately 1774 L. Plasma protein binding of zavegepant is approximately 90%.
Elimination
Metabolism
Zavegepant is primarily metabolized by CYP3A4 and to a lesser extent by CYP2D6, in vitro. After single IV dose of 5 mg [14C]-zavegepant, unchanged zavegepant was the most prevalent (approximately 90%) circulating component in the human plasma. No major metabolites (i.e., greater than 10%) of zavegepant were detected in plasma.
Excretion
The effective half-life of zavegepant following a 10 mg dose of the nasal spray is 6.55 hours. The mean apparent clearance of intranasal zavegepant is 266 L/h. Zavegepant is excreted mostly via the biliary/fecal route, while the renal route is a minor route of elimination. Following a single intravenous dose of 5 mg [14C]-zavegepant to healthy male subjects, approximately 80% and 11% of the dose was recovered as unchanged zavegepant in feces and urine, respectively.
Specific Populations
Patients with Hepatic Impairment
In a dedicated clinical study comparing the pharmacokinetics of zavegepant in subjects with moderate hepatic impairment (Child-Pugh B) to that of normal subjects (matched healthy controls), zavegepant Cmax was 16% higher and AUC was 1.9-fold higher in patients with moderate hepatic impairment. These changes in exposures are not expected to be clinically significant, based on clinical safety experience and minimal accumulation of drug exposures. The impact of severe hepatic impairment (Child-Pugh C) on the pharmacokinetics of zavegepant was not studied [see Use in Specific Populations (8.6)].
Patients with Renal Impairment
The renal route plays a minor role in the clearance of zavegepant. No clinically significant effect on the pharmacokinetics of zavegepant is expected in subjects with estimated creatinine clearance (CLcr) 30 mL/min or greater. In patients with CLcr 15 to 29 mL/min, accumulation of uremic solutes can cause an increase in zavegepant exposures by inhibiting OATP transporters. Zavegepant has not been studied in patients with CLcr less than 15 mL/min [see Use in Specific Populations (8.7)].
Other Specific Populations
Age, sex, race, ethnicity, and body weight did not show clinically significant effects on the pharmacokinetics of zavegepant.
Drug Interaction Studies
In vitro Studies
Enzymes:
Zavegepant is a substrate of CYP3A4 and to a lesser extent CYP2D6. Zavegepant is not an inducer of CYP1A2, 2B6, or 3A4, or an inhibitor of CYP1A2, CYP2C9, 2C19, 2B6, 2D6, 2C8, and 3A4 at clinically relevant concentrations.
Transporters:
Zavegepant is a substrate for OATP1B3 and NTCP (see In vivo studies).
Zavegepant is also a substrate for the transporters P-gp, MATE1, and MATE2-K. Considering the minor contribution of the renal pathway in the clearance of zavegepant, coadministration of zavegepant with inhibitors of P-gp, MATE1, and MATE2-K inhibitors is not expected to result in a clinically significant effect on zavegepant pharmacokinetics.
Zavegepant is not a substrate for BCRP, OATP1B1, OAT1, OAT3, OCT2, BSEP, MRP2, MRP3, and MRP4.
Zavegepant is an inhibitor of OCT2, MATE1, and MATE2-K, but drug interactions for ZAVZPRET are not expected at clinically relevant concentrations. Zavegepant is not an inhibitor of P-gp, BCRP, OAT1, OAT3, OATP1B1, and OATP1B3.
In vivo Studies
CYP3A4 Inhibitors:
Concomitant administration of a single dose of 10 mg ZAVZPRET with itraconazole (a strong CYP3A4 and P-gp inhibitor), at steady state did not result in a clinically relevant effect on the exposures of zavegepant.
OATP1B3 or NTCP Inhibitors:
Concomitant administration of a single oral dose of 100 mg zavegepant with rifampin (an OATP1B3, NTCP inhibitor and a strong CYP3A inducer), at steady state resulted in increased zavegepant exposure (AUC by 2.3-fold and Cmax by 2.2-fold). The observed change in zavegepant exposures is a composite effect of inhibition of OATP1B3 and NTCP transporters as well as induction of CYP3A enzymes. Concomitant administration of ZAVZPRET with inhibitors of OATP1B3 or NTCP transporters may result in a significant increase in zavegepant exposure [see Drug Interactions (7.1)].
OATP1B3 or NTCP Inducers:
Concomitant administration of ZAVZPRET with inducers of OATP1B3 or NTCP transporters has not been studied. However, since zavegepant is a substrate of OATP1B3 and NTCP, concomitant administration with inducers of these transporters may result in decreased zavegepant exposure [see Drug Interactions (7.2)].
Intranasal Decongestants:
The effect of concomitant intranasal decongestants on the pharmacokinetics of zavegepant nasal spray has not been evaluated. Concomitant administration of intranasal decongestants may decrease the systemic exposure of zavegepant and potentially the efficacy of zavegepant [see Drug Interactions (7.3)].
Other Drugs:
No significant pharmacokinetic interactions were observed when zavegepant was concomitantly administered with oral contraceptives (ethinyl estradiol) or sumatriptan [see Clinical Pharmacology (12.2)].
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Intranasal administration of zavegepant (0, 0.3, 0.8, or 2.5 mg/day) to Tg.rasH2 mice for 26 weeks resulted in no evidence of drug-related tumors.
Intranasal administration of zavegepant (0, 2, 9, or 18.8 mg/kg/day) to rats for up to 96 weeks resulted in no evidence of drug-related tumors. Plasma exposure (AUC) at the highest dose tested was approximately 140 times that in humans at the maximum recommended human dose (MRHD) of 10 mg/day.
Mutagenesis
Zavegepant was negative in in vitro (bacterial reverse mutation, chromosomal aberration in Chinese hamster ovary cells) and in vivo (rat micronucleus) assays.
Impairment of Fertility
Subcutaneous administration of zavegepant (0, 5, 15, or 25 mg/kg/day) to male and female rats prior to and during mating and continuing in females to gestation day 7 resulted in no adverse effects on fertility or reproductive performance. Plasma exposures (AUC) at the highest dose tested were approximately 2800 times that in humans at MRHD.
14. Clinical Studies
The efficacy of ZAVZPRET for the acute treatment of migraine with or without aura in adults was demonstrated in two randomized, double-blind, placebo-controlled trials (Study 1 and Study 2). In both studies, patients were instructed to treat a migraine of moderate to severe headache pain intensity. Rescue medication (i.e., NSAIDs, acetaminophen, and/or an antiemetic) was allowed 2 hours after the initial treatment. Other forms of rescue medication such as triptans were not allowed within 48 hours of initial treatment. In Study 1 and Study 2, 13.4% and 13.6% of patients were taking preventive medications for migraine at baseline, respectively. None of the patients were on concomitant preventive medication that act on the CGRP pathway.
In Study 1 (NCT04571060), patients were randomized to receive a single dose of ZAVZPRET 10 mg (N=623) or placebo (N=646). Efficacy was demonstrated with ZAVZPRET 10 mg by an effect on the coprimary endpoints of pain freedom and most bothersome symptom (MBS) freedom at 2 hours after a single dose, compared to placebo. Pain freedom was defined as a reduction of moderate or severe headache pain to no headache pain, and MBS freedom was defined as the absence of the self-identified MBS (i.e., photophobia, phonophobia, or nausea). The most common MBS reported before dosing was photophobia (55%), followed by nausea (28%), and phonophobia (16%).
In Study 1, the percentage of patients achieving headache pain freedom and MBS freedom 2 hours after a single dose was statistically significantly greater in patients who received ZAVZPRET compared to those who received placebo (Table 2).
Table 2. Efficacy Endpoints in Study 1:
| ZAVZPRET 10 mg | Placebo | |
|---|---|---|
| Pain Free at 2 hours | ||
| n/N* | 147/623 | 96/646 |
| % Responders | 23.6 | 14.9 |
| Difference from placebo (%) | 8.8 | |
| p-value | <0.001 | |
| MBS† Free at 2 hours | ||
| n/N* | 247/623 | 201/646 |
| % Responders | 39.6 | 31.1 |
| Difference from placebo (%) | 8.7 | |
| p-value | 0.001 | |
* n = number of responders/N=number of patients in that treatment group
† MBS = most bothersome symptoms of photophobia, phonophobia, or nausea.
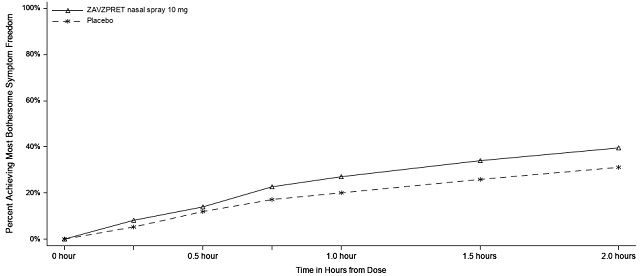
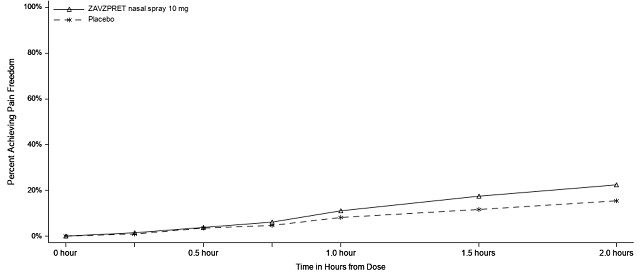
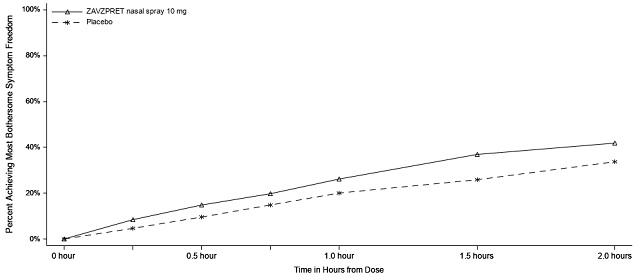
Figures 1 and 2 present the percentage of patients achieving migraine pain freedom and MBS freedom within 2 hours following treatment in Study 1.
Figure 1. Percentage of Patients Achieving Pain Freedom within 2 Hours in Study 1:
Figure 2. Percentage of Patients Achieving MBS Freedom within 2 Hours in Study 1:
In Study 1, statistically significant effects of ZAVZPRET compared to placebo were demonstrated for the additional efficacy endpoints of pain relief at 2 hours post-dose, return to normal function at 2 hours post-dose, sustained pain freedom from 2 to 48 hours post-dose (Table 3), and phonophobia and photophobia freedom at 2 hours post-dose. Pain relief was defined as a reduction in migraine pain from moderate or severe severity to mild or none. The measurement of the percentage of patients reporting normal function at two hours after dosing was derived from a single item questionnaire, asking patients to select one response on a 4-point scale: normal function, mild impairment, severe impairment, or required bedrest.
Table 3. Additional Efficacy Endpoints in Study 1:
| ZAVZPRET 10 mg | Placebo | |
|---|---|---|
| Pain Relief at 2 hours | ||
| n/N* | 366/623 | 321/646 |
| % Responders | 58.7 | 49.7 |
| Difference from placebo (%) | 9.0 | |
| p-value | 0.001 | |
| Percentage of Patients Reporting Normal Function at 2 hours† | ||
| n/N* | 204/570 | 152/593 |
| % Responders | 35.8 | 25.6 |
| Difference from placebo (%) | 10.2 | |
| p-value | <0.001 | |
| Sustained Pain Freedom from 2 to 48 hours | ||
| n/N* | 77/623 | 56/646 |
| % Responders | 12.4 | 8.7 |
| Difference from placebo (%) | 3.7 | |
| p-value | 0.031 | |
* n = number of responders/N=number of patients in that treatment group
† Includes patients with functional disability at time of dosing, according to the functional disability scale.
The incidence of photophobia and phonophobia was reduced following administration of ZAVZPRET 10 mg as compared to placebo.
In Study 2 (NCT03872453), patients were randomized to receive a single dose of ZAVZPRET 10 mg (n=391) or placebo (n=401).
In Study 2, statistically significant efficacy was demonstrated with ZAVZPRET 10 mg by an effect on the coprimary endpoints of pain freedom and most bothersome symptom (MBS) freedom at 2 hours after a single dose, compared to placebo. Pain freedom was observed in 22.5% of patients receiving ZAVZPRET and 15.5% of patients receiving placebo (p-value=0.011). MBS freedom was observed in 41.9% of patients receiving ZAVZPRET and 33.7% of patients receiving placebo (p-value=0.016). The most common MBS reported before dosing was photophobia (53%), followed by nausea (31%), and phonophobia (15%).
Table 4. Efficacy Endpoints in Study 2:
| ZAVZPRET 10 mg | Placebo | |
|---|---|---|
| Pain Free at 2 hours | ||
| n/N* | 88/391 | 62/401 |
| % Responders | 22.5 | 15.5 |
| Difference from placebo (%) | 7.0 | |
| p-value | 0.011 | |
| MBS† Free at 2 hours | ||
| n/N* | 164/391 | 135/401 |
| % Responders | 41.9 | 33.7 |
| Difference from placebo (%) | 8.3 | |
| p-value | 0.016 | |
* n = number of responders/N=number of patients in that treatment group
† MBS = most bothersome symptoms of photophobia, phonophobia, or nausea.
Figure 3. Percentage of Patients Achieving Pain Freedom within 2 Hours in Study 2:
Figure 4. Percentage of Patients Achieving MBS Freedom within 2 Hours in Study 2:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.