ZEPATIER Film-coated tablet Ref.[10450] Active ingredients: Elbasvir Elbasvir and Grazoprevir Grazoprevir
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, The Netherlands
4.3. Contraindications
Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
Patients with moderate or severe hepatic impairment (Child-Pugh B or C) (see sections 4.2 and 5.2).
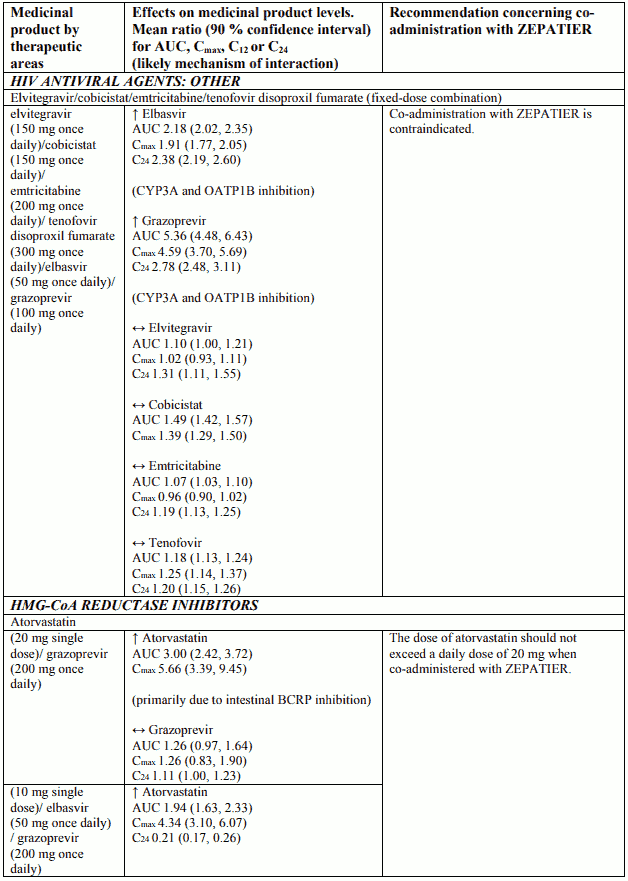
Co-administration with inhibitors of organic anion transporting polypeptide 1B (OATP1B), such as rifampicin, atazanavir, darunavir, lopinavir, saquinavir, tipranavir, cobicistat or ciclosporin (see sections 4.4 and 4.5).
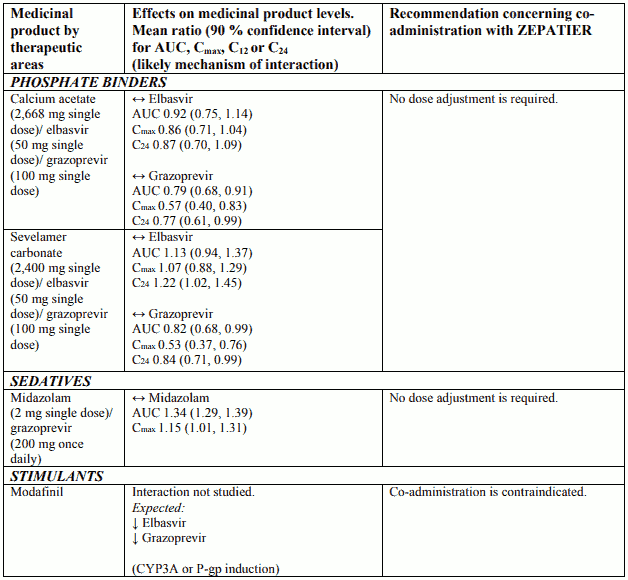
Co-administration with inducers of cytochrome P450 3A (CYP3A) or P-glycoprotein (P-gp), such as efavirenz, phenytoin, carbamazepine, bosentan, etravirine, modafinil or St. John’s wort (Hypericum perforatum) (see sections 4.4 and 4.5).
4.4. Special warnings and precautions for use
ALT elevations
The rate of late ALT elevations during treatment is directly related to the plasma exposure to grazoprevir. During clinical studies with ZEPATIER with or without ribavirin, <1% of subjects experienced elevations of ALT from normal levels to greater than 5 times the upper limit of normal (ULN), (see section 4.8). Higher rates of late ALT elevations occurred in females (2% [11/652]), Asians (2% [4/165]), and subjects aged ≥65 years (2% [3/187]) (see sections 4.8 and 5.2). These late ALT elevations generally occurred at or after treatment week 8.
Hepatic laboratory testing should be performed prior to therapy, at treatment week 8, and as clinically indicated. For patients receiving 16 weeks of therapy, additional hepatic laboratory testing should be performed at treatment week 12.
- Patients should be instructed to consult their healthcare professional without delay if they have onset of fatigue, weakness, lack of appetite, nausea and vomiting, jaundice or discoloured faeces.
- Discontinuation of ZEPATIER should be considered if ALT levels are confirmed to be greater than 10 times the ULN.
- ZEPATIER should be discontinued if ALT elevation is accompanied by signs or symptoms of liver inflammation or increasing conjugated bilirubin, alkaline phosphatase, or international normalised ratio (INR).
Genotype-specific activity
The efficacy of ZEPATIER has not been demonstrated in HCV genotypes 2, 3, 5 and 6. ZEPATIER is not recommended in patients infected with these genotypes.
Retreatment
The efficacy of ZEPATIER in patients previously exposed to ZEPATIER, or to medicinal products of the same classes as those of ZEPATIER (NS5A inhibitors or NS3/4A inhibitors other than telaprevir, simeprevir, boceprevir), has not been demonstrated (see section 5.1).
Interactions with medicinal products
Co-administration of ZEPATIER and OATP1B inhibitors is contraindicated because it may significantly increase grazoprevir plasma concentrations.
Co-administration of ZEPATIER and CYP3A or P-gp inducers is contraindicated because it may significantly decrease elbasvir and grazoprevir plasma concentrations and may lead to a reduced therapeutic effect of ZEPATIER (see sections 4.3, 4.5 and 5.2).
The concomitant use of ZEPATIER and strong CYP3A inhibitors increases elbasvir and grazoprevir concentrations, and co-administration is not recommended (see section 4.5).
HCV/HBV (hepatitis B virus) co-infection
Cases of hepatitis B virus (HBV) reactivation, some of them fatal, have been reported during or after treatment with direct-acting antiviral agents. HBV screening should be performed in all patients before initiation of treatment. HBV/HCV co-infected patients are at risk of HBV reactivation, and should therefore be monitored and managed according to current clinical guidelines.
Use in diabetic patients
Diabetics may experience improved glucose control potentially resulting in symptomatic hypoglycaemia, after initiating HCV direct acting antiviral (DAA) treatment. Glucose levels of diabetic patients initiating DAA therapy should be closely monitored, particularly within the first 3 months, and their diabetic medication modified when necessary. The physician in charge of the diabetic care of the patient should be informed when DAA therapy is initiated.
Paediatric population
ZEPATIER is not indicated for use in children under 12 years of age.
Excipients
ZEPATIER contains lactose monohydrate. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency, or glucose-galactose malabsorption should not take this medicinal product.
ZEPATIER contains 69.85 mg sodium per tablet, equivalent to 3.5% of the WHO recommended maximum daily intake of 2 g sodium for an adult.
4.5. Interaction with other medicinal products and other forms of interaction
Potential for other medicinal products to affect ZEPATIER
Grazoprevir is a substrate of OATP1B drug transporters. Co-administration of ZEPATIER with medicinal products that inhibit OATP1B transporters is contraindicated because it may result in a significant increase in the plasma concentration of grazoprevir (see sections 4.3 and 4.4).
Elbasvir and grazoprevir are substrates of CYP3A and P-gp. Co-administration of inducers of CYP3A or P-gp with ZEPATIER is contraindicated because it may decrease elbasvir and grazoprevir plasma concentrations, which may lead to reduced therapeutic effect of ZEPATIER (see sections 4.3 and 4.4).
Co-administration of ZEPATIER with strong CYP3A inhibitors increases elbasvir and grazoprevir plasma concentrations, and co-administration is not recommended (see Table 2 and section 4.4).
Co-administration of ZEPATIER with P-gp inhibitors is expected to have a minimal effect on the plasma concentrations of ZEPATIER.
The potential for grazoprevir to be a breast cancer resistance protein (BCRP) substrate cannot be excluded.
Potential for ZEPATIER to affect other medicinal products
Elbasvir and grazoprevir are inhibitors of the drug transporter BCRP at the intestinal level in humans and may increase plasma concentrations of co-administered BCRP substrates. Elbasvir is not a CYP3A inhibitor in vitro and grazoprevir is a weak CYP3A inhibitor in humans. Co-administration with grazoprevir did not result in clinically relevant increases in exposures of CYP3A substrates. Therefore, no dose adjustment is required for CYP3A substrates when co-administered with ZEPATIER.
Elbasvir has minimal intestinal P-gp inhibition in humans, and does not result in clinically relevant increases in concentrations of digoxin (a P-gp substrate), with an 11% increase in plasma AUC. Grazoprevir is not a P-gp inhibitor based on in vitro data. Elbasvir and grazoprevir are not OATP1B inhibitors in humans. Based on in vitro data, clinically significant interactions with ZEPATIER as an inhibitor of other CYP enzymes, UGT1A1, esterases (CES1, CES2, and CatA), OAT1, OAT3, and OCT2 are not expected. Based on in vitro data, a potential for GZR to inhibit BSEP cannot be excluded. Multiple-dose administration of elbasvir or grazoprevir is unlikely to induce the metabolism of medicinal products metabolised by CYP isoforms based on in vitro data.
Patients treated with vitamin K antagonists
As liver function may change during treatment with ZEPATIER, a close monitoring of International Normalised Ratio (INR) values is recommended.
Impact of DAA therapy on drugs metabolized by the liver
Grazoprevir’s weak inhibition of CYP3A may increase levels of CYP3A substrates. In addition, the plasma concentrations of drugs that are CYP3A substrates may be decreased by improvement in liver function during DAA therapy, related to clearance of HCV. Therefore, close monitoring and potential dose adjustment of CYP3A substrates with a narrow therapeutic index (e.g., calcineurin inhibitors) may be required during therapy, as drug levels may change (see Table 2).
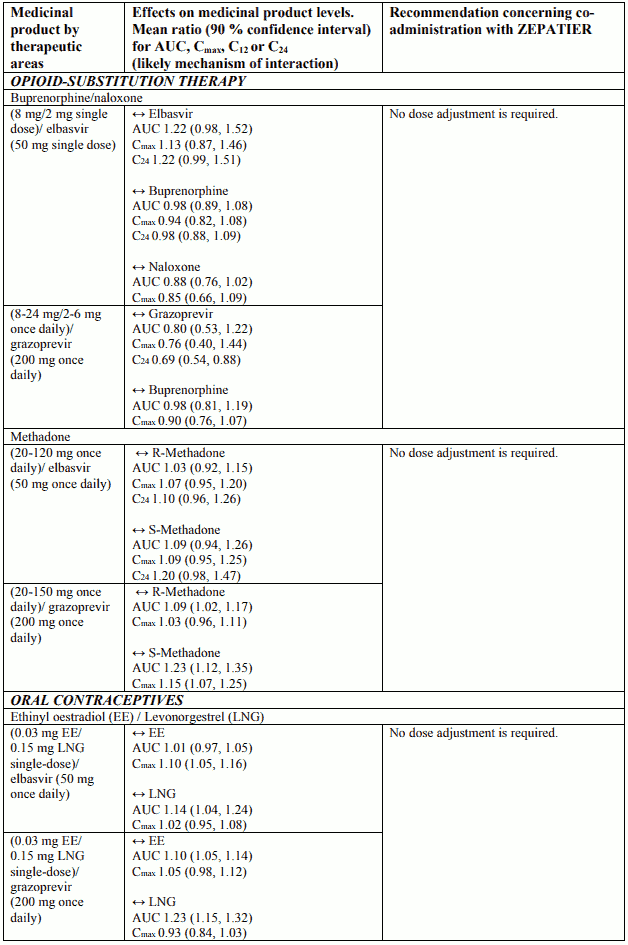
Interactions between ZEPATIER and other medicinal products
Table 2 provides a listing of assessed or potential medicinal product interactions. An up “↑” or down “↓” arrow represents a change in exposure that requires monitoring or a dose adjustment of that medication, or the co-administration is not recommended or contraindicated. No clinically relevant change in exposure is represented by a horizontal arrow “↔”.
The medicinal product interactions described are based on results from studies conducted with either ZEPATIER or elbasvir (EBR) and grazoprevir (GZR) as individual agents, or are predicted medicinal product interactions that may occur with elbasvir or grazoprevir. The table is not all-inclusive.
Table 2. Interactions and dose recommendations with other medicinal products:
Paediatric population
Interaction studies have only been performed in adults.
4.6. Fertility, pregnancy and lactation
If ZEPATIER is co-administered with ribavirin, the information for ribavirin with regard to contraception, pregnancy testing, pregnancy, breast-feeding, and fertility also applies to this combination regimen (refer to the Summary of Product Characteristics for the co-administered medicinal product for additional information).
Women of childbearing potential/contraception in males and females
When ZEPATIER is used in combination with ribavirin, women of childbearing potential or their male partners must use an effective form of contraception during treatment and for a period of time after the treatment has concluded.
Pregnancy
There are no adequate and well-controlled studies with ZEPATIER in pregnant women. Animal studies do not indicate harmful effects with respect to reproductive toxicity. Because reproduction animal studies are not always predictive of human response, ZEPATIER should be used only if the potential benefit justifies the potential risk to the fetus.
Breast-feeding
It is unknown whether elbasvir or grazoprevir and their metabolites are excreted in human milk. Available pharmacokinetic data in animals has shown excretion of elbasvir and grazoprevir in milk. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from ZEPATIER therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman.
Fertility
No human data on the effect of elbasvir and grazoprevir on fertility are available. Animal studies do not indicate harmful effects of elbasvir or grazoprevir on fertility at elbasvir and grazoprevir exposures higher than the exposure in humans at the recommended clinical dose (see section 5.3).
4.7. Effects on ability to drive and use machines
ZEPATIER (administered alone or in combination with ribavirin) is not likely to have an effect on the ability to drive and use machines. Patients should be informed that fatigue has been reported during treatment with ZEPATIER (see section 4.8).
4.8. Undesirable effects
Summary of the safety profile
The safety of ZEPATIER was assessed based on 3 placebo-controlled studies and 7 uncontrolled Phase 2 and 3 clinical studies in approximately 2,000 subjects with chronic hepatitis C infection with compensated liver disease (with or without cirrhosis).
In clinical studies, the most commonly reported adverse reactions (greater than 10%) were fatigue and headache. Less than 1% of subjects treated with ZEPATIER with or without ribavirin had serious adverse reactions (abdominal pain, transient ischaemic attack and anaemia). Less than 1% of subjects treated with ZEPATIER with or without ribavirin permanently discontinued treatment due to adverse reactions. The frequency of serious adverse reactions and discontinuations due to adverse reactions in subjects with compensated cirrhosis were comparable to those seen in subjects without cirrhosis.
When elbasvir/grazoprevir was studied with ribavirin, the most frequent adverse reactions to elbasvir/grazoprevir + ribavirin combination therapy were consistent with the known safety profile of ribavirin.
Tabulated summary of adverse reactions
The following adverse reactions were identified in patients taking ZEPATIER without ribavirin for 12 weeks. The adverse reactions are listed below by body system organ class and frequency. Frequencies are defined as follows: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1,000) or very rare (<1/10,000).
Table 3. Adverse reactions identified with ZEPATIER*:
| Frequency | Adverse reactions |
|---|---|
| Metabolism and nutrition disorders | |
| Common | decreased appetite |
| Psychiatric disorders | |
| Common | insomnia, anxiety, depression |
| Nervous system disorders | |
| Very common | headache |
| Common | dizziness |
| Gastrointestinal disorders | |
| Common | nausea, diarrhoea, constipation, upper abdominal pain, abdominal pain, dry mouth, vomiting |
| Skin and subcutaneous tissue disorders | |
| Common | pruritus, alopecia |
| Musculoskeletal and connective tissue disorders | |
| Common | arthralgia, myalgia |
| General disorders and administration site conditions | |
| Very | common fatigue |
| Common | asthenia, irritability |
* Based on pooled data from patients treated with ZEPATIER for 12 weeks without ribavirin.
Description of selected adverse reactions
Laboratory abnormalities
Changes in selected laboratory parameters are described in Table 4.
Table 4. Selected treatment emergent laboratory abnormalities:
| Laboratory Parameters | ZEPATIER* N=834 n (%) |
|---|---|
| ALT (IU/L) | |
| 5.1-10.0 × ULN† (Grade 3) | 6 (0.7%) |
| >10.0 × ULN (Grade 4) | 6 (0.7%) |
| Total Bilirubin (mg/dL) | |
| 2.6-5.0 × ULN (Grade 3) | 3 (0.4%) |
| >5.0 × ULN (Grade 4) | 0 |
* Based on pooled data from patients treated with ZEPATIER for 12 weeks without ribavirin
† ULN: Upper limit of normal according to testing laboratory
Serum Late ALT elevations
During clinical studies with ZEPATIER with or without ribavirin, regardless of treatment duration, <1% (13/1,690) of subjects experienced elevations of ALT from normal levels to greater than 5 times the ULN, generally at or after treatment week 8 (mean onset time 10 weeks, range 6-12 weeks). These late ALT elevations were typically asymptomatic. Most late ALT elevations resolved with ongoing therapy with ZEPATIER or after completion of therapy (see section 4.4). The frequency of late ALT elevations was higher in subjects with higher grazoprevir plasma concentration (see sections 4.4, 4.5 and 5.2). The incidence of late ALT elevations was not affected by treatment duration. Cirrhosis was not a risk factor for late ALT elevations. Less than 1% of subjects treated with ZEPATIER with or without ribavirin experienced ALT elevations >2.5 – 5 times the ULN during treatment; there were no treatment discontinuations due to these ALT elevations.
Paediatric population
The safety assessment of Zepatier in paediatric patients aged 12 years and older is based on data from a Phase 2b, open-label clinical study that enrolled 22 patients who were treated with Zepatier for 12 weeks.The adverse reactions observed were consistent with those observed in clinical studies of Zepatier in adults.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.