Panobinostat
Chemical formula: C₂₁H₂₃N₃O₂ Molecular mass: 349.434 g/mol PubChem compound: 6918837
Interactions
Panobinostat interacts in the following cases:
CYP3A inhibitors, Pgp inhibitors
Panobinostat metabolism is through both non-CYP and CYP mediated routes. Approximately 40% of panobinostat is metabolised through CYP3A4. Therefore, medicinal products that can influence CYP3A4 enzyme activity may alter the pharmacokinetics of panobinostat. Panobinostat is a P-gp substrate.
Co-administration of a single 20 mg panobinostat dose with ketoconazole, a strong CYP3A inhibitor, increased the Cmax and AUC of panobinostat by 1.6- and 1.8-fold, respectively, compared to when panobinostat was given alone.
In patients who take concomitant medicinal products which are strong CYP3A and/or Pgp inhibitors, including, but not limited to, ketoconazole, itraconazole, voriconazole, ritonavir, saquinavir, telithromycin, posaconazole and nefazodone, the dose of panobinostat should be reduced.
Patients should be instructed to avoid star fruit, grapefruit, grapefruit juice, pomegranates and pomegranate juice, as these are known to inhibit cytochrome P450 3A enzymes and may increase the bioavailability of panobinostat.
CYP3A4 substrates
No data is available that can be used to exclude the risk that panobinostat could be a weak inducer of the enzyme CYP3A4 in the gastrointestinal tract. This could potentially lead to slightly decreased exposure to sensitive CYP3A4 substrates.
Hepatotoxicity, hepatic impairment
Hepatic dysfunction, primarily mild transient elevations in aminotransferases and total bilirubin, has been reported in patients during treatment with panobinostat.
Liver function should be monitored prior to treatment and regularly during treatment. If results of liver function tests show abnormalities according to the NCI-CTEP classification, dose adjustments for patients with mild and moderate hepatic impairment are recommended and the patient should be followed until values return to normal or pre-treatment levels. Panobinostat should not be administered in patients with severe hepatic impairment due to lack of experience and safety data in this population. Adjustment of bortezomib dose should also be considered.
A clinical study in cancer patients with impaired hepatic function showed that plasma exposure of panobinostat increased by 43% (1.4-fold) and 105% (2-fold) in patients with mild and moderate hepatic impairment, respectively. Patients with mild hepatic impairment should be started on panobinostat at a reduced dose of 15 mg during the first treatment cycle. A dose escalation from 15 mg to 20 mg may be considered based on patient tolerability. Patients with moderate hepatic impairment should be started on panobinostat at a reduced dose of 10 mg during the first treatment cycle. A dose escalation from 10 mg to 15 mg may be considered based on patient tolerability. Frequency of monitoring of these patients should be increased during treatment with panobinostat, particularly during the dose escalation phase. Panobinostat should not be administered in patients with severe hepatic impairment due to lack of experience and safety data in this population. Adjustment of bortezomib dose should also be considered.
Recommended starting dose modification for patients with hepatic impairment:
| Grade of hepatic impairment* | Bilirubin level | SGOT (AST) levels | Modification of panobinostat starting dose | Modification of bortezomib starting dose |
|---|---|---|---|---|
| Mild | ≤1.0 x ULN | >ULN | Reduce panobinostat dose to 15 mg in the first treatment cycle. Consider dose escalation up to 20 mg in subsequent cycles based on patient tolerability. | None. |
| >1.0 x ULN και ≤1.5 x ULN | Any | |||
| Moderate | >1.5 x ULN και ≤3.0 x ULN | Any | Reduce panobinostat dose to 10 mg in the first treatment cycle. Consider dose escalation up to 15 mg in subsequent cycles based on patient tolerability. | Reduce bortezomib dose to 0.7 mg/m² in the first treatment cycle. Consider dose escalation to 1.0 mg/m² or further dose reduction to 0.5 mg/m² in subsequent cycles based on patient tolerability. |
SGOT = serum glutamic oxaloacetic transaminase;
AST = aspartate aminotransferase
ULN = upper limit of the normal range
* Based on NCI-CTEP classification
Strong CYP3A4 inducers
Strong inducers may reduce the efficacy of panobinostat, therefore the concomitant use of strong CYP3A4 inducers including, but not limited to, carbamazepine, phenobarbital, phenytoin, rifabutin, rifampicin and St. John’s Wort (Hypericum perforatum), should be avoided.
CYP2D6 substrates
Panobinostat increased the Cmax and the AUC of dextromethorphan (a substrate of CYP2D6) by 1.8- and 1.6-fold, respectively, and it cannot be excluded that the effect may be larger on a more sensitive CYP2D6 substrate. Avoid panobinostat use in patients who are taking CYP2D6 substrates with a narrow therapeutic index (including, but not limited to, pimozide). When panobinostat is co-administered with sensitive CYP2D6 substrates (e.g. atomoxetine, dextromethorphan, metoprolol, nebivolol, perphenazine and pimozide), dose titrate individual CYP2D6 substrates based on tolerability and frequently monitor patients for adverse reactions.
CYP3A4 inducers
The panobinostat fraction metabolised through CYP3A4 is approximately 40%. In clinical studies in multiple myeloma, the exposure of panobinostat was decreased by approximately 20% by the concomitant use of dexamethasone, which is a dose-dependent mild/moderate CYP3A4 inducer. Strong inducers are expected to have greater effects, and may reduce the efficacy of panobinostat, therefore the concomitant use of strong CYP3A4 inducers including, but not limited to, carbamazepine, phenobarbital, phenytoin, rifabutin, rifampicin and St. John’s Wort (Hypericum perforatum), should be avoided.
Fertility
Based on non-clinical findings, male fertility may be compromised by treatment with panobinostat.
Dolasetron, granisetron, ondansetron, tropisetron
Anti-emetic medicinal products with a known risk of QT prolongation such as dolasetron, granisetron, ondansetron and tropisetron should be used with caution.
Hypothyroidism
Hypothyroidism events were reported in 8 of 381 patients treated with panobinostat + bortezomib + dexamethasone in Study D2308, of whom 2 required treatment. Thyroid and pituitary function should be monitored by measuring hormone levels (e.g. free T4 and TSH) as clinically indicated.
Haemorrhage, coagulation disorders, chronic anti-coagulation therapy
Haemorrhage has been reported in patients during treatment with panobinostat. CTC grade 3 or 4 haemorrhage was reported in 4.2% of patients, including cases of gastrointestinal and pulmonary haemorrhage with fatal outcomes. Therefore, physicians and patients should be aware of the increased risk of thrombocytopenia and the potential for haemorrhage, especially in patients with coagulation disorders or in those who are receiving chronic anti-coagulation therapy.
Thrombocytopenia, neutropenia, anaemia
Haematological adverse drug reactions, including severe thrombocytopenia, neutropenia and anaemia (CTC grade 3 to 4) were reported in patients treated with panobinostat. Therefore a complete blood count must be performed before initiating therapy with panobinostat, with frequent monitoring during treatment (in particular before each injection of bortezomib as per bortezomib SmPC).
The platelet count should be ≥100 × 109/l and the absolute neutrophil count ≥1.0 × 109/l prior to initiation of treatment. Platelet count should be ≥100 × 109/l prior to initiating any cycle of treatment.
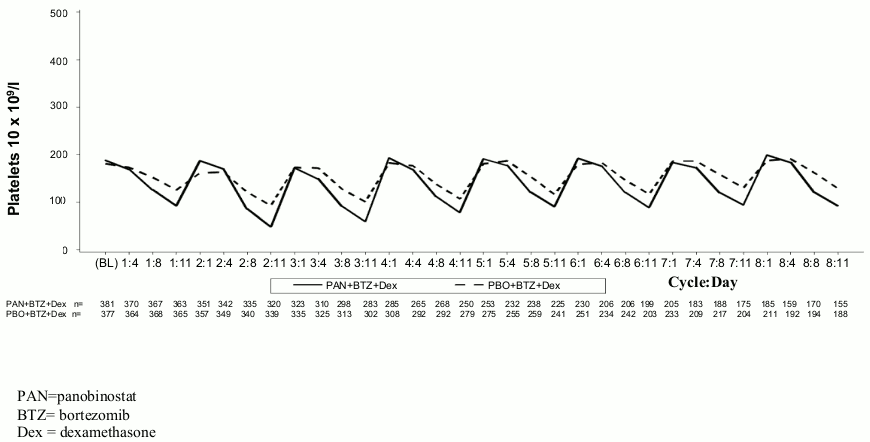
In the phase III study, thrombocytopenia typically recovered to baseline by the start of the next 21-day cycle (see Figure 1). The median time to onset for grade 3 and 4 thrombocytopenia was one month and the median time to recovery was 12 days.
Figure 1. Median platelet counts over time (Study D2308, Safety set, cycles 1-8):
In patients with CTC grade 3 thrombocytopenia (platelet count <50 × 109/l with bleeding) panobinostat may need to be temporarily withheld and/or the subsequent dose may need to be reduced. Platelet transfusions may be required as clinically indicated.
Dose adjustments
Modification of the treatment dose and/or schedule may be required based on individual tolerability. Clinical judgement on how to continue the treatment should be exercised when a patient experiences an adverse drug reaction.
If a dose reduction is required, the dose of panobinostat should be reduced by decrements of 5 mg (i.e. from 20 mg to 15 mg or from 15 mg to 10 mg). The dose should not be reduced below 10 mg and the same treatment schedule (3-week treatment cycle) should be kept.
Thrombocytopenia
Platelet counts should be monitored prior to each dose of bortezomib (i.e. on days 1, 4, 8 and 11 of cycles 1-8 and on days 1 and 8 of cycles 9-16). If patients experience thrombocytopenia, panobinostat may need to be temporarily withheld and the subsequent dose may need to be reduced (see Table 1). In patients with platelet count <50 × 109/l (complicated by bleeding) or <25 × 109/l, panobinostat therapy should be withheld and resumed at a reduced dose upon recovery to platelet count ≥50 × 109/l. Platelet counts should be monitored at least twice a week until ≥50 × 109/l. Platelet transfusions may be required, if clinically indicated. Discontinuation of treatment may be considered if thrombocytopenia does not improve despite the treatment modifications described below and/or the patient requires repeated platelet transfusions. Additionally, dose adjustment of bortezomib may be considered (see bortezomib SmPC and Table 1).
Table 1. Recommended dose modifications for thrombocytopenia:
| Thrombocytopenia grade on day of treatment | Modification of panobinostat starting dose | Panobinostat dose on recovery to grade 2 thrombocytopenia (≥50 × 109/l) | Modification of bortezomib starting dose | Bortezomib dose on recovery to grade 2 thrombocytopenia (≥50 × 109/l) | |
|---|---|---|---|---|---|
| 1 dose omitted | More than 1 dose omitted | ||||
| Grade 3 Platelets <50 × 109/l with bleeding | Omit dose | Resume at reduced dose | Omit dose | Resume at same dose | Resume at reduced dose |
| Βαθμού 4 Αιμοπετάλια <25 × 109/l | Omit dose | Resume at reduced dose | Omit dose | Resume at same dose | Resume at reduced dose |
Neutropenia
Neutropenia may require temporary or permanent dose reduction. Instructions for dose interruptions and reductions for panobinostat are outlined in Table 2.
Table 2. Recommended dose modifications for neutropenia:
| Neutropenia grade on day of treatment | Modification of panobinostat starting dose | Panobinostat dose on recovery to grade 2 neutropenia (<1.5-1.0 × 109/l) | Modification of bortezomib starting dose | Bortezomib dose on recovery to grade 2 neutropenia (<1.5-1.0 × 109/l) |
|---|---|---|---|---|
| Grade 3 neutropenia (<1.0-0.5 × 109/l) | Omit dose | Resume at same dose | Omit dose | Resume at same dose |
| Grade 4 neutropenia (<0.5 × 109/l) or febrile neutropenia (<1.0 × 109/l and fever ≥38.5°C) | Omit dose | Resume at reduced dose | Omit dose | Resume at same dose |
In the event of grade 3 or 4 neutropenia, physicians should consider the use of growth factors (e.g. G-CSF) according to local guidelines. Discontinuation of treatment may be considered if neutropenia does not improve despite the dose modifications and/or despite the addition of granulocyte colony stimulating factor therapy according to local medical practice and treatment guidelines, and/or in the event of severe secondary infections.
Infection
Localised and systemic infections, including pneumonia, other bacterial infections, invasive fungal infections such as aspergillosis or candidiasis, and viral infections including hepatitis B virus and herpes simplex, have been reported in patients taking panobinostat. Some of these infections (e.g. pneumonia) have been severe (e.g. leading to sepsis, or respiratory or multi-organ failure) and have had fatal outcomes. Of note, whereas grade 3 and grade 4 neutropenia were observed in 28% and 7% of patients, respectively, febrile neutropenia was observed in 1% of patients. Physicians and patients should be aware of the increased risk of infection with panobinostat.
Panobinostat treatment should not be initiated in patients with active infections. Pre-existing infections should be treated prior to initiation of the therapy. Patients should be monitored for signs and symptoms of infections during treatment with panobinostat; if a diagnosis of infection is made, appropriate anti-infective treatment should be instituted promptly and interruption or discontinuation of panobinostat considered.
If a diagnosis of invasive systemic fungal infection is made, panobinostat should be discontinued and appropriate anti-fungal therapy instituted.
Nausea, diarrhoea, constipation, vomiting
Severe nausea, diarrhoea, constipation and vomiting, sometimes requiring the use of anti-emetic and anti-diarrhoeal medicinal products, have been reported in patients treated with panobinostat. Fluid and electrolyte blood levels, especially potassium, magnesium and phosphate, should be monitored periodically during therapy and corrected as clinically indicated to prevent potential dehydration and electrolyte disturbances.
Prophylactic anti-emetics (e.g. prochlorperazine) may be considered at the discretion of the physician and in accordance with local medical practice. Anti-emetic medicinal products with a known risk of QT prolongation such as dolasetron, granisetron, ondansetron and tropisetron should be used with caution.
At the first sign of abdominal cramping, loose stools or onset of diarrhoea, it is recommended that the patient be treated with anti-diarrhoeal medicinal product (e.g. loperamide) or any additional treatment in accordance with local treatment guidelines. Replacement intravenous fluids and electrolytes may be used as appropriate. Medicinal products with laxative properties should be used with caution because of the potential for exacerbation of diarrhoea. Patients should be advised to contact their physician to discuss the use of any laxative product.
Gastrointestinal toxicity is very common in patients treated with panobinostat. Patients who experience diarrhoea and nausea or vomiting may require temporary dose discontinuation or dose reduction as outlined in the following table.
Recommended dose modifications for gastrointestinal toxicity:
| Adverse drug reaction | Grade on day of treatment | Modification of panobinostat starting dose | Panobinostat dose on recovery to ≤ grade 1 | Modification of bortezomib starting dose | Bortezomib dose on recovery to ≤ grade 1 |
|---|---|---|---|---|---|
| Diarrhoea | Grade 2 despite anti-diarrhoeal medicinal product | Omit dose | Resume at the same dose | Omit dose | Resume at reduced dose or change to once weekly |
| Grade 3 despite anti-diarrhoeal medicinal product | Omit dose | Resume at reduced dose | Omit dose | Resume at reduced dose or with the same dose but with a once-weekly schedule | |
| Grade 4 despite anti-diarrhoeal medicinal product | Permanently discontinue | Permanently discontinue |
At the first sign of abdominal cramping, loose stools or onset of diarrhoea, it is recommended that the patient be treated with an anti-diarrhoeal medicinal product (e.g. loperamide).
In the event of grade 3 nausea or grade 3 or 4 vomiting despite administration of an anti-emetic, panobinostat should be temporarily discontinued and resumed at a reduced dose on recovery to grade 1.
Prophylactic anti-emetics should be administered at the discretion of the physician and in accordance with local medical practice.
Drugs that prolong the QT interval, long QT syndrome, recent myocardial infarction, bradycardia
Based on preclinical and clinical data, panobinostat has the potential to prolong the QT interval. Concomitant use of anti-arrhythmic medicinal products (including, but not limited to, amiodarone, disopyramide, procainamide, quinidine and sotalol) and other substances that are known to prolong the QT interval (including, but not limited to, chloroquine, halofantrine, clarithromycin, methadone, moxifloxacin, bepridil and pimozide) is not recommended. Anti-emetic medicinal products with a known risk of QT prolongation such as dolasetron, granisetron, ondansetron and tropisetron should be used with caution.
Panobinostat may prolong cardiac ventricular repolarisation (QT interval).
No episodes of QTcF prolongation >500 msec were reported with the dose of 20 mg panobinostat in the phase III clinical study, in combination with bortezomib and dexamethasone. Pooled clinical data from over 500 patients treated with panobinostat alone in multiple indications and at different dose levels have shown that the incidence of CTC grade 3 QTc prolongation (QTcF >500 msec) was approximately 1% overall and 5% or more at a dose of 60 mg or higher; no episodes of torsades de pointes were observed.
Additional analysis suggests that the risk of QTc prolongation does not increase over time.
QTcF should be <480 msec prior to initiation of treatment with panobinostat.
Appropriate monitoring of electrolytes (e.g. potassium, magnesium and phosphorus) and ECG should be performed at baseline and periodically during treatment, particularly in patients with severe gastrointestinal adverse drug reaction.
Panobinostat should be used with caution in patients who already have or who are at significant risk of developing QTc prolongation. This includes patients:
- with long QT syndrome.
- with uncontrolled or significant cardiac disease, including recent myocardial infarction, congestive heart failure, unstable angina or clinically significant bradycardia.
Concomitant administration of medicinal products that are known to cause QTc prolongation should be used with caution.
In case of concomitant use of agents that may increase panobinostat plasma concentrations, such as strong CYP3A4 inhibitors, dose adjustment is required.
In the event of long QT interval prior to initiation of panobinostat (QTcF ≥480 msec at baseline), the start of treatment should be delayed until pre-dose average QTcF has returned to <480 msec. In addition any abnormal serum potassium, magnesium or phosphorus values should be corrected prior to initiation of panobinostat therapy. In the event of QT prolongation during treatment:
- The dose should be omitted, if QTcF is ≥480 msec or above 60 msec from baseline.
- If QT prolongation is resolved within 7 days, resume treatment at prior dose for initial occurrence or at reduced dose if QT prolongation is recurrent.
- If QT prolongation is unresolved within 7 days, treatment should be discontinued.
- If any QTcF value is above 500 msec, panobinostat therapy should be permanently discontinued.
Pregnancy
There are no clinical studies on the use of panobinostat in pregnant patients. Studies in animals have shown reproductive and embryo-foetal toxicity. Given panobinostat’s cytostatic/cytotoxic mode of action, the potential risk to the foetus is high. Panobinostat should only be used during pregnancy if the expected benefits outweigh the potential risks to the foetus. If it is used during pregnancy or if the patient becomes pregnant while using it, the patient must be informed of the potential risk to the foetus.
Nursing mothers
It is unknown whether panobinostat is excreted in human milk. Given its cytostatic/cytotoxic mode of action, breast-feeding is contraindicated during panobinostat treatment.
Carcinogenesis, mutagenesis and fertility
Women of child-bearing potential/Contraception in males and females
Based on findings in animals, the likelihood of panobinostat increasing the risk of both foetal death and developmental skeletal abnormalities when administered to pregnant women is predicted to be high. Women of child-bearing potential should have a pregnancy test prior to the initiation of treatment with panobinostat and must use a highly effective method of contraception during treatment and for three months after the last dose of panobinostat. Women using hormonal contraceptives should additionally use a barrier method of contraception.
Due to its cytostatic/cytotoxic mode of action, panobinostat can influence the quality of sperm formed during treatment. Sexually active men taking panobinostat and their female partners should use a highly effective method of contraception during the man’s treatment and for six months after his last dose of panobinostat.
When panobinostat is administered together with dexamethasone, which is known to be a weak to moderate inducer of CYP3A4 as well as other enzymes and transporters, the risk for reduced efficacy of hormonal contraceptives needs to be considered. In addition, it is currently unknown whether panobinostat may reduce the effectiveness of hormonal contraceptives, and therefore women using hormonal contraceptives should additionally use a barrier method of contraception.
Fertility
Based on non-clinical findings, male fertility may be compromised by treatment with panobinostat.
Effects on ability to drive and use machines
Panobinostat has a minor influence on the ability to drive and use machines. Dizziness may occur following administration of panobinostat.
Adverse reactions
Summary of the safety profile
The safety data of panobinostat have been assessed from a total of 451 patients with multiple myeloma treated with panobinostat in combination with bortezomib and dexamethasone and from a total of 278 patients treated with panobinostat as a single agent.
The safety data reported below are based on the phase III clinical study (Panorama 1) in 381 patients with multiple myeloma treated with 20 mg panobinostat once a day three times per week, on a 2 weeks on and 1 week off dosing regimen in combination with bortezomib and dexamethasone. The median duration of exposure in the study was 5.0 months. 15.7% of patients were exposed to study treatment for ≥48 weeks.
The most common non-haematological adverse reactions were diarrhoea, fatigue, nausea and vomiting.
Treatment-emergent haematological toxicities included thrombocytopenia, anaemia, neutropenia and lymphopenia.
QTcF >480 and <500 msec was recorded in 1.3% of patients and change from baseline of >60 msec was observed in 0.8% of patients. No patient had an absolute QTcF >500 msec.
Cardiac events (most frequently atrial fibrillation, tachycardia, palpitation and sinus tachycardia) were reported in 17.6% of panobinostat + bortezomib + dexamethasone-treated patients versus 9.8% of placebo + bortezomib + dexamethasone-treated patients and syncope events were reported in 6.0% versus 2.4%, respectively.
Discontinuation due to adverse events, regardless of causality, was observed in 36.2% of patients. The most common adverse events (AEs) leading to treatment discontinuation were diarrhoea (4.5%), asthenia and fatigue (2.9% each) and pneumonia (1.3%).
On-treatment deaths not due to the study indication (multiple myeloma) were reported in 6.8% of panobinostat + bortezomib + dexamethasone-treated patients versus 3.2% of placebo + bortezomib + dexamethasone-treated patients.
List of adverse drug reactions from clinical studies
Adverse drug reactions from the phase III study (Panorama 1) are shown in the list below. Adverse drug reactions are listed according to system organ classes in MedDRA. Within each system organ class, the adverse drug reactions are ranked by frequency, with the most frequent reactions first. Within each frequency grouping, adverse drug reactions are presented in order of decreasing seriousness. In addition, the corresponding frequency category for each adverse drug reaction is based on the following convention (CIOMS III): very common (≥1/10); common (≥1/100 to <1/10); uncommon (≥1/1,000 to <1/100); rare (≥1/10,000 to <1/1,000); very rare (<1/10,000); and not known (cannot be estimated from available data).
The following list includes adverse drug reactions that occur due to the addition of panobinostat to the bortezomib and dexamethasone combination. The frequency category reflects the combination of all the medicinal products i.e. panobinostat + bortezomib + dexamethasone. For adverse drug reactions that are related to bortezomib or dexamethasone treatment, please refer to the relevant SmPC.
Panobinostat adverse drug reactions observed in multiple myeloma patients in the phase III study:
Infections and infestations
Very common: Upper respiratory tract infection, pneumonia
Common: Septic shock, urinary tract infection, viral infection, oral herpes, Clostridium difficile colitis, otitis media, cellulitis, sepsis, gastroenteritis, lower respiratory tract infection, candidiasis
Uncommon: Pneumonia fungal, hepatitis B, aspergillosis
Blood and lymphatic system disordersa
Very common: Pancytopenia, thrombocytopenia, anaemia, leukopenia, neutropenia, lymphopenia
Endocrine disorders
Common: Hypothyroidism
Metabolism and nutrition disorders
Very common: Decreased appetite, hypophosphataemiaa, hyponatraemiaa, hypokalaemiaa
Common: Hyperglycaemia, dehydration, hypoalbuminaemia, fluid retention, hyperuricaemia, hypocalcaemia, hypomagnesaemia
Psychiatric disorders
Very common: Insomnia
Nervous system disorders
Very common: Dizziness, headache
Common: Haemorrhage intracranial, syncope, tremor, dysgeusia
Eye disorders
Common: Conjunctival haemorrhage
Cardiac disorders
Common: Bradycardia, atrial fibrillation, sinus tachycardia, tachycardia, palpitation
Uncommon: Myocardial infarction
Vascular disorders
Very common: Hypotension
Common: Hypertension, haematoma, orthostatic hypotension
Uncommon: Shock haemorrhagic
Respiratory, thoracic and mediastinal disorders
Very common: Cough, dyspnoea
Common: Respiratory failure, rales, wheezing, epistaxis
Uncommon: Pulmonary haemorrhage, haemoptysis
Gastrointestinal disorders
Very common: Diarrhoea, nausea, vomiting, abdominal pain, dyspepsia
Common: Gastrointestinal haemorrhage, haematochezia, gastritis, cheilitis, abdominal distension, dry mouth, flatulence
Uncommon: Colitis, haematemesis, gastrointestinal pain
Hepatobiliary disorders
Common: Hepatic function abnormal, hyperbilirubinaemiaa
Skin and subcutaneous disorders
Common: Skin lesions, rash, erythema
Uncommon: Petechiae
Musculoskeletal and connective tissue disorders
Common: Joint swelling
Renal and urinary disorders
Common: Renal failure, haematuria, urinary incontinence
General disorders and administration site conditions
Very common: Fatigue, oedema peripheral, pyrexia, asthenia
Common: Chills, malaise
Investigations
Very common: Weight decreased
Common: Blood urea increased, glomerular filtration rate decreased, blood alkaline phosphatase increased, electrocardiogram QT prolonged, blood creatinine increaseda, SGPT alanine transamina__se (ALT) increaseda, SGOT aspartate transaminase (AST) increaseda
a Frequency is based on laboratory values
Description of selected adverse drug reactions
Gastrointestinal
Gastrointestinal toxicity, primarily diarrhoea, nausea and vomiting, is among the most frequently reported adverse reactions. However, treatment discontinuation due to these reactions was reported in a relatively small proportion of patients, with diarrhoea at 4.5% and nausea and vomiting at 0.5% each. Patients should be advised to contact their physician if severe gastrointestinal toxicity occurs and dose adjustment or discontinuation may be required.
Thrombocytopenia
Due to the nature of multiple myeloma and the known haematotoxicity for panobinostat and its combination agent bortezomib, thrombocytopenia, often severe, has been frequently observed. CTC grade 3 or 4 thrombocytopenia occurred in 256 patients, with a median onset time of one month. However, thrombocytopenia is reversible (median time to recovery of 12 days) and can usually be managed by dose adjustment and interruption with or without platelet transfusion. 33.3% patients in the panobinostat + bortezomib + dexamethasone arm and 10.3% patients in the placebo + bortezomib + dexamethasone arm received platelet transfusions during treatment.
Thrombocytopenia rarely leads to treatment discontinuation (1.6% of patients). Most patients with thrombocytopenia did not experience haemorrhage. 20.7% of patients experienced haemorrhage, most frequently epistaxis (4.7%), haematoma (2.6%), and conjunctival haemorrhage (2.1%). CTC grade 3 or 4 haemorrhage was reported in 4.2% of patients, mostly commonly involving gastrointestinal haemorrhage. Five patients (1.3%) died of events associated with haemorrhage. Amongst the patients who died of haemorrhage, one patient had thrombocytopenia grade 4, three patients had thrombocytopenia grade 3 and 1 patient had thrombocytopenia grade 1.
Neutropenia
Neutropenia was frequently reported on the basis of laboratory findings determined during the study (all grades: 75%). Most newly occurring severe neutropenia was grade 3 (28%), with considerably fewer cases of grade 4 (6.6%). While many patients developed neutropenia, febrile neutropenia only occurred in a fraction of treated patients (1.0%, both for CTC all grades and for grades 3 and 4). Patients with neutropenia are prone to infection, mostly upper respiratory tract infection or pneumonia. Only 0.3% of the patients were discontinued from the treatment due to neutropenia.
Fatigue and asthenia
Fatigue and asthenia were reported in 41.2% and 22.0% of patients, respectively. CTC grade 3 fatigue was reported in 15.7% of the patients, and grade 4 in 1.3%. Grade 3 asthenia was observed in 9.4% of the patients, with no patients experiencing asthenia at CTC grade 4. The treatment was discontinued in 2.9% of patients due to fatigue and asthenia.
Infections
Relapsed or refractory multiple myeloma patients are at risk of infections. Potential contributing factors may include prior history of chemotherapy, stem cell transplant, the nature of the disease and neutropenia or lymphopenia associated with panobinostat treatment. The most frequently reported infections include upper respiratory tract infection, pneumonia and nasopharyngitis. Fatalities involving either pneumonia or sepsis were reported. Treatment discontinuation due to infections was reported in 5% of patients.
QT prolongation and ECG abnormalities
QTc prolongation was observed and was mostly mild in degree: QTcF interval >450 msec and ≤480 msec was reported in 10.8% of patients, with maximum increase from baseline >30 msec and ≤60 msec in 14.5% of patients. QTcF >500 msec was not reported in any patient.
ECG (electrocardiogram) abnormalities have been reported in patients treated with panobinostat + bortezomib + dexamethasone, mainly involving ST-T depression (21.7%) and T wave changes (39.6%). Regardless of events chronology, syncope was reported in 9% of patients with ST-T depression and 7.2% of patients with T wave change and 4.9% of patients with neither of these ECG abnormalities. Likewise ischaemic heart disease (including myocardial infarction and ischaemia) were reported in 4.5% of patients with ST-T depression and 4.8% of patients with T wave change and 2.7% of patients with neither of these ECG abnormalities.
Special populations
Elderly population
The incidence of deaths not related to study indication was 8.8% in patients ≥65 years of age compared to 5.4% in patients <65 years of age.
Adverse reactions leading to permanent discontinuation occurred in 30%, 44% and 47% of patients aged <65 years, 65-75 years and ≥75 years, respectively. Grade 3-4 events more frequently observed in patients included the following (percentages presented for patients <65 years, 65-75 years and ≥75 years of age, respectively): thrombocytopenia (60%, 74%, and 91%), anaemia (16%, 17% and 29%), diarrhoea (21%, 27% and 47%), and fatigue (18%, 28% and 47%).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.