Pramlintide
Chemical formula: C₁₇₁H₂₆₇N₅₁O₅₃S₂ Molecular mass: 3,949.44 g/mol
Pharmacodynamic properties
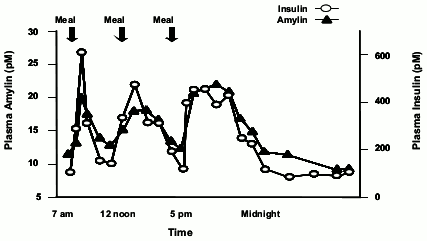
Pramlintide is an analog of human amylin. Amylin is colocated with insulin in secretory granules and cosecreted with insulin by pancreatic beta cells in response to food intake. Amylin and insulin show similar fasting and postprandial patterns in healthy individuals (Figure 1).
Figure 1. Secretion Profile of Amylin and Insulin in Healthy Adults:
In patients with type 1 and type 2 diabetes, there is reduced secretion from pancreatic beta cells of both insulin and amylin in response to food.
Amylin affects the rate of postprandial glucose appearance through a variety of mechanisms, as determined by nonclinical studies. Amylin slows gastric emptying (i.e. the rate at which food is released from the stomach to the small intestine) without altering the overall absorption of nutrients. In addition, amylin suppresses glucagon secretion (not normalized by insulin alone), which leads to suppression of endogenous glucose output from the liver. Amylin also regulates food intake due to centrally-mediated modulation of appetite.
In human studies, pramlintide, acting as an amylin analog, slows gastric emptying, reduces the postprandial rise in plasma glucagon, and modulates satiety leading to decreased caloric intake.
Pharmacokinetic properties
Absorption
The absolute bioavailability of pramlintide following a single subcutaneous is approximately 30% to 40%. Subcutaneous administration of different doses of pramlintide into the abdominal area or thigh of healthy individuals showed a linear, dose-dependent increase in maximum plasma concentrations (Cmax) and overall exposure (AUC) (Table 2).
Table 2. Mean Pharmacokinetic Parameters Following Administration of Single Subcutaneous Doses of pramlintide:
| Subcutaneous Dose (mcg) | AUC0-∞ (pmol*min/L) | Cmax (pmol/L) | Tmax (min) | Elimination t1⁄2 (min) |
|---|---|---|---|---|
| 30 | 3750 | 39 | 21 | 55 |
| 60 | 6778 | 79 | 20 | 49 |
| 90 | 8507 | 102 | 19 | 51 |
| 120 | 11970 | 147 | 21 | 48 |
Injection of pramlintide into the arm in obese patients with type 1 or type 2 diabetes showed higher overall exposure (20%-36%) with greater variability (% CV for AUC: 73%-106%), compared with exposure after injection of pramlintide into the abdominal area or thigh.
Relative bioavailability of pramlintide was not significantly different between obese and non-obese patients and based on BMI or skin fold thickness. Injections administered with 6.0-mm and 12.7-mm needles yielded similar bioavailability.
Distribution
Pramlintide does not extensively bind to red blood cells or albumin (approximately 40% of the drug is unbound in plasma).
Metabolism and Elimination In healthy individuals, the half-life of pramlintide is approximately 48 minutes. The primary metabolite, Des-lys1 pramlintide (2-37 pramlintide), is biologically active in vitro. Overall exposure (AUC) to pramlintide is relatively constant with repeat dosing of pramlintide, indicating no bioaccumulation.
Specific Populations
Renal Impairment
No studies have been conducted in patients with end-stage renal disease. In a single-dose pharmacokinetic study in patients with type 1 diabetes, 60 mcg of pramlintide was administered to 4 patients with normal renal function (ClCr >90 mL/min), 9 patients with mild renal impairment (ClCr 60-89 mL/min), 5 patients with moderate renal impairment (ClCr 30-59 mL/min) and 3 patients with severe renal impairment (ClCr 15-29 mL/min). No statistically significant differences were noted in total (AUC0-∞) and peak (Cmax) exposure of pramlintide for mild, moderate, and severe renal impairment categories in comparison to patients with normal renal function; although, inter-patient variability in pharmacokinetic parameters was high.
Hepatic Impairment
Pharmacokinetic studies have not been conducted in patients with hepatic impairment.
Geriatric
Pharmacokinetic studies have not been conducted in the geriatric population.
Pediatric
The efficacy and safety of pramlintide have not been established in the pediatric population. The use of pramlintide is not recommended in pediatric patients due to the risk of severe hypoglycemia.
Gender
No study has been conducted to evaluate the effect of gender on pramlintide pharmacokinetics.
Race/Ethnicity
No study has been conducted to evaluate the effect of ethnicity on pramlintide pharmacokinetics.
Drug Interactions
Effect of Pre-Mixing Pramlintide with Insulin
Pharmacokinetic profiles of pramlintide and insulins after coadministration of 30 mcg pramlintide with different insulins (regular, NPH, and 70/30 premixed formulations of recombinant human insulin) as one subcutaneous injection, premixed in one syringe, were compared to those observed after the coadministration of pramlintide and different insulins given as separate subcutaneous injections. The effects of premixing on pramlintide pharmacokinetics varied across the different insulin products with a maximum decrease of 40% in pramlintide Cmax and a maximum increase of 36% in pramlintide AUC0-∞. Similarly, effects of premixing on insulin pharmacokinetics varied across different insulin products with a maximum increase of 15% in insulin Cmax and up to a 20% increase in insulin AUC0-600min. Always administer pramlintide and insulin as separate injections and never mix.
Acetaminophen
When 1000 mg acetaminophen was given within 0, 1, and 2 hours after a 120 mcg pramlintide injection in patients with type 2 diabetes (n=24), acetaminophen Cmax decreased by 29%, 23%, and 20%, respectively compared to placebo. The time to maximum plasma concentration or Tmax increased by 72, 48, and 48 minutes, respectively. Pramlintide did not significantly affect acetaminophen Tmax or Cmax when acetaminophen was administered 1 to 2 hours before pramlintide injection. Pramlintide did not affect acetaminophen AUC regardless of the time of acetaminophen administration in relation to pramlintide injection.
Oral Contraceptives
When a single dose of a combination oral contraceptive product, containing 30 mcg ethinyl estradiol and 300 mcg norgestrel, was administered 15 minutes after pramlintide injection (90 mcg dose) in healthy female subjects, there was no statistically significant change in the Cmax and AUC of ethinyl estradiol. However, the norgestrel Cmax was reduced by about 30% and Tmax was delayed by 45 minutes; there was no effect on norgestrel AUC. The clinical relevance of this change is unknown.
Ampicillin
The effect of concomitant administration of pramlintide and ampicillin was evaluated in healthy volunteers. The administration of a single oral 500 mg dose of ampicillin 15 minutes after a single dose of pramlintide (90 mcg) did not alter the Cmax or AUC for ampicillin. However, the Tmax for ampicillin was delayed by approximately 60 minutes.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.