EYLEA 114.3 mg/ml Solution for injection Ref.[110823] Active ingredients: Aflibercept
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Bayer AG, 51368 Leverkusen, Germany
4.1. Therapeutic indications
Eylea is indicated in adults for the treatment of
- neovascular (wet) age-related macular degeneration (nAMD) (see section 5.1)
- visual impairment due to diabetic macular oedema (DME) (see section 5.1).
4.2. Posology and method of administration
Eylea must only be administered by a qualified physician experienced in intravitreal injections.
Posology
The recommended dose is 8 mg aflibercept, equivalent to 0.07 ml solution. The posology is the same for the nAMD and DME indications. The 8 mg dose requires use of the Eylea 114.3 mg/ml vial.
Eylea treatment is initiated with 1 injection per month for 3 consecutive doses. Injection intervals may then be extended up to every 4 months based on the physician’s judgement of visual and/or anatomic outcomes. Subsequently, the treatment intervals may be further extended up to 5 months, such as with a treat-and-extend dosing regimen, while maintaining stable visual and/or anatomic outcomes (see section 5.1).
If visual and/or anatomic outcomes deteriorate, the treatment interval should be shortened accordingly based on the physician’s discretion. The shortest interval between 2 injections is 2 months in the maintenance phase.
Eylea at monthly doses of 8 mg has not been studied for more than 3 consecutive doses.
The frequency of monitoring visits should be based on the patient’s status and at the physician’s discretion. For events in which treatment should be withheld see section 4.4.
Special populations
Renal or hepatic impairment
No specific studies in patients with renal or hepatic impairment have been conducted. Available data do not suggest a need for a dose adjustment with Eylea in these patients (see section 5.2).
Elderly
Available data do not suggest a need for a dose adjustment with Eylea in these patients.
Paediatric population
The safety and efficacy of Eylea 114.3 mg/ml in children and adolescents below 18 years have not been established. There is no relevant use of Eylea 114.3 mg/ml in the paediatric population in the nAMD and DME indications.
Method of administration
Eylea is for intravitreal injection only.
Intravitreal injections must be carried out according to medical standards and applicable guidelines by a qualified physician experienced in administering intravitreal injections. In general, adequate anaesthesia and asepsis, including topical broad spectrum microbicide (e.g. povidone iodine applied to the periocular skin, eyelid and ocular surface), have to be ensured. Surgical hand disinfection, sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent) are recommended.
The injection needle should be inserted 3.5 to 4.0 mm posterior to the limbus into the vitreous cavity, avoiding the horizontal meridian and aiming towards the centre of the globe. The injection volume of 0.07 ml is then delivered. A different scleral site should be used for subsequent injections.
Immediately following the intravitreal injection, patients should be monitored for elevation in intraocular pressure. Appropriate monitoring may consist of a check for perfusion of the optic nerve head or tonometry. If required, sterile equipment for paracentesis should be available.
Following intravitreal injection, patients should be instructed to report any symptoms suggestive of endophthalmitis (e.g. eye pain, redness of the eye, photophobia, blurring of vision) without delay.
Each vial should only be used for the treatment of a single eye. After injection, discard any unused product or waste material in accordance with local requirements.
For handling of the medicinal product before administration, see section 6.6.
4.9. Overdose
Overdosing with increased injection volume may increase intraocular pressure. Therefore, in case of overdose, intraocular pressure should be monitored and, if deemed necessary by the treating physician, adequate treatment should be initiated (see sections 4.4 and 6.6).
6.3. Shelf life
2 years.
6.4. Special precautions for storage
Store in a refrigerator (2°C–8°C).
Do not freeze.
Keep the vial in the outer carton in order to protect from light.
The unopened vial may be stored outside the refrigerator below 25°C for up to 24 hours.
6.5. Nature and contents of container
Vial (type I glass) with a grey rubber stopper (chlorobutyl) sealed with an aluminium cap with white lid, and a 18 G, 5-micron filter needle.
Each vial contains 0.263 ml solution.
Pack size of 1 vial and 1 filter needle.
6.6. Special precautions for disposal and other handling
The vial is for single use in one eye only. Extraction of multiple doses from a single vial may increase the risk of contamination and subsequent infection.
Do not use if the package or its components are expired, damaged, or have been tampered with. Check the label on the vial to make sure you have the strength of Eylea that you intended to use. The 8 mg dose requires use of the Eylea 114.3 mg/ml vial.
18 G, 5-micron filter needle:
- BD blunt filter (fill) needle, not for skin injection.
- Do not autoclave BD blunt filter (fill) needle.
- The filter needle is non-pyrogenic. Do not use it if individual packaging is damaged.
- Discard the used BD Blunt Filter (Fill) Needle in approved sharps collector.
- Caution: Re-use of the filter needle may lead to infection or other illness/injury.
The intravitreal injection should be performed with a 30 G × ½ inch injection needle (not included). Use of a smaller size needle (higher gauge) than the recommended 30 G × ½ inch injection needle may result in increased injection forces.
1. Prior to administration visually inspect the solution for injection. Do not use the vial if particulates, cloudiness, or discolouration are visible.
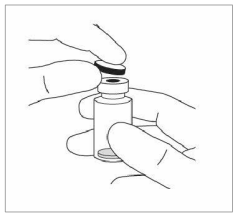
2. Remove the plastic cap and disinfect the outer part of the rubber stopper of the vial.
3. Use aseptic technique to carry out steps 3-10. Attach the filter needle supplied in the carton to a 1-ml sterile, Luer-lock syringe.
4. Push the filter needle into the centre of the vial stopper until the needle is completely inserted into the vial and the tip touches the bottom or bottom edge of the vial.
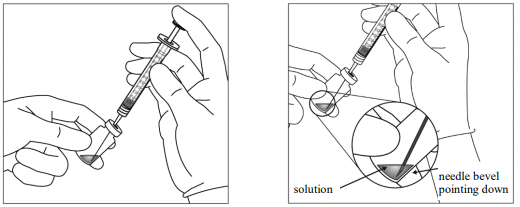
5. Withdraw all of the Eylea vial content into the syringe, keeping the vial in an upright position, slightly inclined to ease complete withdrawal. To deter the introduction of air, ensure the bevel of the filter needle is submerged into the liquid. Continue to tilt the vial during withdrawal keeping the bevel of the filter needle submerged in the liquid.
6. Ensure that the plunger rod is drawn sufficiently back when emptying the vial to completely empty the filter needle. After injection any unused product must be discarded.
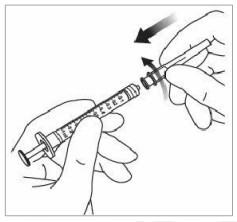
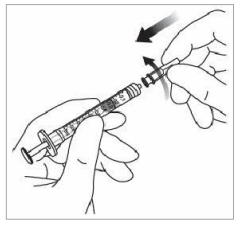
7. Remove the filter needle and properly dispose of it.
Note: The filter needle is not to be used for the intravitreal injection.
8. Firmly twist the 30 G × ½ inch injection needle onto the Luer-lock syringe tip.
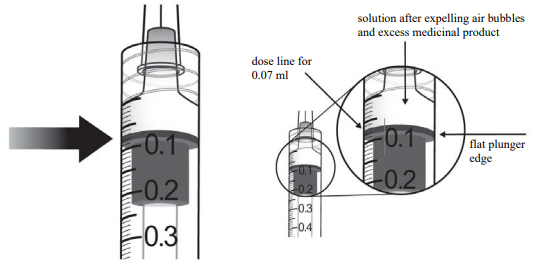
9. Holding the syringe with the needle pointing up, check the syringe for bubbles. If there are bubbles, gently tap the syringe with your finger until the bubbles rise to the top.
10. To eliminate all bubbles and to expel excess medicinal product, slowly depress the plunger so that the flat plunger edge aligns with the line that marks 0.07 ml on the syringe.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.