ACIPHEX Delayed-release tablet Ref.[10530] Active ingredients: Rabeprazole
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Rabeprazole belongs to a class of antisecretory compounds (substituted benzimidazole proton-pump inhibitors) that do not exhibit anticholinergic or histamine H2-receptor antagonist properties, but suppress gastric acid secretion by inhibiting the gastric H, K ATPase at the secretory surface of the gastric parietal cell. Because this enzyme is regarded as the acid (proton) pump within the parietal cell, rabeprazole has been characterized as a gastric proton-pump inhibitor. Rabeprazole blocks the final step of gastric acid secretion.
In gastric parietal cells, rabeprazole is protonated, accumulates, and is transformed to an active sulfenamide. When studied in vitro, rabeprazole is chemically activated at pH 1.2 with a half-life of 78 seconds. It inhibits acid transport in porcine gastric vesicles with a half-life of 90 seconds.
12.2. Pharmacodynamics
Antisecretory Activity
The antisecretory effect begins within one hour after oral administration of 20 mg ACIPHEX delayed-release tablets. The median inhibitory effect of rabeprazole on 24 hour gastric acidity is 88% of maximal after the first dose. A 20 mg dose of ACIPHEX delayed-release tablets inhibits basal and peptone meal-stimulated acid secretion versus placebo by 86% and 95%, respectively, and increases the percent of a 24-hour period that the gastric pH>3 from 10% to 65% (see table below). This relatively prolonged pharmacodynamic action compared to the short pharmacokinetic half-life (1 to 2 hours) reflects the sustained inactivation of the H, K ATPase.
Table 3. Gastric Acid Parameters: ACIPHEX Delayed-Release Tablets versus Placebo After 7 Days of Once Daily Dosing:
| Parameter | ACIPHEX delayed-release tablets (20 mg once daily) | Placebo |
|---|---|---|
| Basal Acid Output (mmol/hr) | 0.4* | 2.8 |
| Stimulated Acid Output (mmol/hr) | 0.6* | 13.3 |
| % Time Gastric pH>3 | 65* | 10 |
* (p<0.01 versus placebo)
Compared to placebo, 10 mg, 20 mg, and 40 mg of ACIPHEX delayed-release tablets, administered once daily for 7 days significantly decreased intragastric acidity with all doses for each of four meal-related intervals and the 24-hour time period overall. In this study, there were no statistically significant differences between doses; however, there was a significant dose-related decrease in intragastric acidity. The ability of rabeprazole to cause a dose-related decrease in mean intragastric acidity is illustrated below.
Table 4. AUC Acidity (Mmol•Hr/L): ACIPHEX Delayed-Release Tablets versus Placebo on Day 7 of Once Daily Dosing (Mean±SD):
| ACIPHEX delayed-release tablets | Placebo (N=24) | |||
|---|---|---|---|---|
| AUC interval (hrs) | 10 mg (N=24) | 20 mg (N=24) | 40 mg (N=24) | |
| 08:00 – 13:00 | 19.6±21.5* | 12.9±23* | 7.6±14.7* | 91.1±39.7 |
| 13:00 – 19:00 | 5.6±9.7* | 8.3±29.8* | 1.3±5.2* | 95.5±48.7 |
| 19:00 – 22:00 | 0.1±0.1* | 0.1±0.06* | 0.0±0.02* | 11.9±12.5 |
| 22:00 – 08:00 | 129.2±84* | 109.6±67.2* | 76.9±58.4* | 479.9±165 |
| AUC 0-24 hours | 155.5±90.6* | 130.9±81* | 85.8±64.3* | 678.5±216 |
* (p<0.001 versus placebo)
After administration of 20 mg ACIPHEX delayed-release tablets once daily for eight days, the mean percent of time that gastric pH greater than 3 or gastric pH greater than 4 after a single dose (Day 1) and multiple doses (Day 8) was significantly greater than placebo (see table below). The decrease in gastric acidity and the increase in gastric pH observed with 20 mg ACIPHEX delayed-release tablets administered once daily for eight days were compared to the same parameters for placebo, as illustrated below:
Table 5. Gastric Acid Parameters ACIPHEX Delayed-Release Tablets Once Daily Dosing versus Placebo on Day 1 and Day 8:
| Parameter | ACIPHEX delayed-release tablets 20 mg once daily | Placebo | ||
|---|---|---|---|---|
| Day 1 | Day 8 | Day 1 | Day 8 | |
| Mean AUC0-24 Acidity | 340.8* | 176.9* | 925.5 | 862.4 |
| Median trough pH (23-hr)a | 3.77 | 3.51 | 1.27 | 1.38 |
| % Time Gastric pH greater than 3b | 54.6* | 68.7* | 19.1 | 21.7 |
| % Time Gastric pH greater than 4b | 44.1* | 60.3* | 7.6 | 11.0 |
a No inferential statistics conducted for this parameter.
b Gastric pH was measured every hour over a 24-hour period.
* (p<0.001 versus placebo)
Effects on Esophageal Acid Exposure
In patients with GERD and moderate to severe esophageal acid exposure, a dose of 20 mg and 40 mg per day of ACIPHEX delayed-release tablets decreased 24-hour esophageal acid exposure. After seven days of treatment, the percentage of time that the esophageal pH was less than 4 decreased from baselines of 24.7% for 20 mg and 23.7% for 40 mg, to 5.1% and 2.0%, respectively. Normalization of 24-hour intraesophageal acid exposure was correlated to gastric pH greater than 4 for at least 35% of the 24-hour period; this level was achieved in 90% of subjects receiving ACIPHEX 20 mg and in 100% of subjects receiving ACIPHEX 40 mg. With ACIPHEX 20 mg and 40 mg per day, significant effects on gastric and esophageal pH were noted after one day of treatment, and more pronounced after seven days of treatment.
Effects on Serum Gastrin
The median fasting gastrin level increased in a dose-related manner in patients treated once daily with ACIPHEX delayed-release tablets for up to eight weeks for ulcerative or erosive esophagitis and in patients treated for up to 52 weeks to prevent recurrence of disease. The group median values stayed within the normal range.
In a group of subjects treated with 20 mg ACIPHEX delayed-release tablets for 4 weeks a doubling of mean serum gastrin concentrations was observed. Approximately 35% of these treated subjects developed serum gastrin concentrations above the upper limit of normal.
Effects on Enterochromaffin-like (ECL) Cells
Increased serum gastrin secondary to antisecretory agents stimulates proliferation of gastric ECL cells which, over time, may result in ECL cell hyperplasia in rats and mice and gastric carcinoids in rats, especially in females [see Nonclinical Toxicology (13.1)].
In over 400 patients treated with ACIPHEX delayed-release tablets (10 or 20 mg) once daily for up to one year, the incidence of ECL cell hyperplasia increased with time and dose, which is consistent with the pharmacological action of the proton pump inhibitor. No patient developed the adenomatoid, dysplastic or neoplastic changes of ECL cells in the gastric mucosa. No patient developed the carcinoid tumors observed in rats.
Endocrine Effects
Studies in humans for up to one year have not revealed clinically significant effects on the endocrine system. In healthy male subjects treated with ACIPHEX delayed-release tablets for 13 days, no clinically relevant changes have been detected in the following endocrine parameters examined: 17 β-estradiol, thyroid stimulating hormone, tri-iodothyronine, thyroxine, thyroxine-binding protein, parathyroid hormone, insulin, glucagon, renin, aldosterone, follicle-stimulating hormone, luteotrophic hormone, prolactin, somatotrophic hormone, dehydroepiandrosterone, cortisol-binding globulin, and urinary 6β-hydroxycortisol, serum testosterone and circadian cortisol profile.
Other Effects
In humans treated with ACIPHEX delayed-release tablets for up to one year, no systemic effects have been observed on the central nervous, lymphoid, hematopoietic, renal, hepatic, cardiovascular, or respiratory systems. No data are available on long-term treatment with ACIPHEX delayed-release tablets and ocular effects.
12.3. Pharmacokinetics
After oral administration of 20 mg ACIPHEX delayed-release tablets, peak plasma concentrations (Cmax) of rabeprazole occur over a range of 2 to 5 hours (Tmax). The rabeprazole Cmax and AUC are linear over an oral dose range of 10 mg to 40 mg. There is no appreciable accumulation when doses of 10 mg to 40 mg are administered every 24 hours; the pharmacokinetics of rabeprazole is not altered by multiple dosing.
Absorption
Absolute bioavailability for a 20 mg oral tablet of rabeprazole (compared to intravenous administration) is approximately 52%. When ACIPHEX delayed-release tablets are administered with a high fat meal, Tmax is variable; which concomitant food intake may delay the absorption up to 4 hours or longer. However, the Cmax and the extent of rabeprazole absorption (AUC) are not significantly altered. Thus ACIPHEX delayed-release tablets may be taken without regard to timing of meals.
Distribution
Rabeprazole is 96.3% bound to human plasma proteins.
Elimination
Metabolism
Rabeprazole is extensively metabolized. A significant portion of rabeprazole is metabolized via systemic nonenzymatic reduction to a thioether compound. Rabeprazole is also metabolized to sulphone and desmethyl compounds via cytochrome P450 in the liver. The thioether and sulphone are the primary metabolites measured in human plasma. These metabolites were not observed to have significant antisecretory activity. In vitro studies have demonstrated that rabeprazole is metabolized in the liver primarily by cytochromes P450 3A (CYP3A) to a sulphone metabolite and cytochrome P450 2C19 (CYP2C19) to desmethyl rabeprazole. CYP2C19 exhibits a known genetic polymorphism due to its deficiency in some sub-populations (e.g., 3 to 5% of Caucasians and 17 to 20% of Asians). Rabeprazole metabolism is slow in these sub-populations, therefore, they are referred to as poor metabolizers of the drug.
Excretion
Following a single 20 mg oral dose of 14C-labeled rabeprazole, approximately 90% of the drug was eliminated in the urine, primarily as thioether carboxylic acid; its glucuronide, and mercapturic acid metabolites. The remainder of the dose was recovered in the feces. Total recovery of radioactivity was 99.8%. No unchanged rabeprazole was recovered in the urine or feces.
Specific Populations
Geriatric Patients
In 20 healthy elderly subjects administered 20 mg ACIPHEX delayed-release tablets once daily for seven days, AUC values approximately doubled and the Cmax increased by 60% compared to values in a parallel younger control group. There was no evidence of drug accumulation after once daily administration [see Use in Specific Population (8.5)].
Pediatric Patients
The pharmacokinetics of rabeprazole was studied in 12 adolescent patients with GERD 12 to 16 years of age, in a multicenter study. Patients received 20 mg ACIPHEX delayed-release tablets once daily for five or seven days. An approximate 40% increase in rabeprazole exposure was noted following 5 to 7 days of dosing compared with the exposure after 1 day dosing. Pharmacokinetic parameters in adolescent patients with GERD 12 to 16 years of age were within the range observed in healthy adult subjects.
Male and Female Patients and Racial or Ethnic Groups
In analyses adjusted for body mass and height, rabeprazole pharmacokinetics showed no clinically significant differences between male and female subjects. In studies that used different formulations of rabeprazole, AUC0-∞ values for healthy Japanese men were approximately 50 to 60% greater than values derived from pooled data from healthy men in the United States.
Patients with Renal Impairment
In 10 patients with stable end-stage renal disease requiring maintenance hemodialysis (creatinine clearance ≤5 mL/min/1.73 m 2), no clinically significant differences were observed in the pharmacokinetics of rabeprazole after a single 20 mg dose of ACIPHEX delayed-release tablets when compared to 10 healthy subjects.
Patients with Hepatic Impairment
In a single dose study of 10 patients with mild to moderate hepatic impairment (Child-Pugh Class A and B, respectively) who were administered a single 20 mg dose of ACIPHEX delayed-release tablets, AUC0-24 was approximately doubled, the elimination half-life was 2- to 3-fold higher, and total body clearance was decreased to less than half compared to values in healthy men.
In a multiple dose study of 12 patients with mild to moderate hepatic impairment administered 20 mg ACIPHEX delayed-release tablets once daily for eight days, AUC0-∞ and Cmax values increased approximately 20% compared to values in healthy age- and gender-matched subjects. These increases were not statistically significant.
No information exists on rabeprazole disposition in patients with severe hepatic impairment (Child-Pugh Class C) [see Use in Specific Populations (8.6)].
Drug Interaction Studies
Combined Administration with Antimicrobials
Sixteen healthy subjects genotyped as extensive metabolizers with respect to CYP2C19 were given 20 mg ACIPHEX delayed-release tablets, 1000 mg amoxicillin, 500 mg clarithromycin, or all 3 drugs in a four-way crossover study. Each of the four regimens was administered twice daily for 6 days. The AUC and Cmax for clarithromycin and amoxicillin were not different following combined administration compared to values following single administration. However, the rabeprazole AUC and Cmax increased by 11% and 34%, respectively, following combined administration. The AUC and Cmax for 14-hydroxyclarithromycin (active metabolite of clarithromycin) also increased by 42% and 46%, respectively. This increase in exposure to rabeprazole and 14-hydroxyclarithromycin is not expected to produce safety concerns.
Effects of Other Drugs on Rabeprazole
Antacids: Co-administration of ACIPHEX delayed-release tablets and antacids produced no clinically relevant changes in plasma rabeprazole concentrations.
Effects of Rabeprazole on Other Drugs
Studies in healthy subjects have shown that rabeprazole does not have clinically significant interactions with other drugs metabolized by the CYP450 system, such as theophylline (CYP1A2) given as single oral doses, diazepam (CYP2C9 and CYP3A4) as a single intravenous dose, and phenytoin (CYP2C9 and CYP2C19) given as a single intravenous dose (with supplemental oral dosing). Steady state interactions of rabeprazole and other drugs metabolized by this enzyme system have not been studied in patients.
Clopidogrel: Clopidogrel is metabolized to its active metabolite in part by CYP2C19. A study of healthy subjects including CYP2C19 extensive and intermediate metabolizers receiving once daily administration of clopidogrel 75 mg concomitantly with placebo or with 20 mg ACIPHEX delayed-release tablets (n=36), for 7 days was conducted. The mean AUC of the active metabolite of clopidogrel was reduced by approximately 12% (mean AUC ratio was 88%, with 90% CI of 81.7 to 95.5%) when ACIPHEX delayed-release tablets were coadministered compared to administration of clopidogrel with placebo [seeDrug Interactions (7)].
Digoxin: In healthy adult subjects (n=16), co-administration of 20 mg rabeprazole sodium delayed-release tablets with 2.5 mg once daily doses of digoxin at steady state resulted in approximately 29% and 19% increase in mean Cmax and AUC(0-24) of digoxin [see Drug Interactions (7)].
Ketoconazole: In healthy adult subjects (n=19), co-administration of 20 mg rabeprazole sodium delayed-release tablets at steady state with a single 400 mg oral dose ketoconazole resulted in approximately an average of 31% reduction in both Cmax and AUC(0-inf) of ketoconazole [see Drug Interactions (7)].
Cyclosporine: In vitro incubations employing human liver microsomes indicated that rabeprazole inhibited cyclosporine metabolism with an IC50 of 62 micromolar, a concentration that is over 50 times higher than the Cmax in healthy volunteers following 14 days of dosing with 20 mg of ACIPHEX delayed-release tablets. This degree of inhibition is similar to that by omeprazole at equivalent concentrations.
12.4. Microbiology
The following in vitro data are available but the clinical significance is unknown.
Rabeprazole sodium, amoxicillin and clarithromycin as a three drug regimen has been shown to be active against most strains of Helicobacter pylori in vitro and in clinical infections [see Indications and Usage (1), Clinical Studies (14.5)].
Helicobacter pylori
Susceptibility testing of H. pylori isolates was performed for amoxicillin and clarithromycin using agar dilution methodology1, and minimum inhibitory concentrations (MICs) were determined.
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures.
Incidence of Antibiotic-Resistant Organisms Among Clinical Isolates
Pretreatment Resistance
Clarithromycin pretreatment resistance rate (MIC ≥1 mcg/mL) to H. pylori was 9% (51/560) at baseline in all treatment groups combined. Greater than 99% (558/560) of patients had H. pylori isolates which were considered to be susceptible (MIC ≤0.25 mcg/mL) to amoxicillin at baseline. Two patients had baseline H. pylori isolates with an amoxicillin MIC of 0.5 mcg/mL.
For susceptibility testing information about Helicobacter pylori, see Microbiology section in prescribing information for clarithromycin and amoxicillin.
Table 6. Clarithromycin Susceptibility Test Results and Clinical/ Bacteriologic Outcomes a for a Three Drug Regimen (ACIPHEX Delayed-Release Tablets 20 mg Twice Daily, Amoxicillin 1000 mg Twice Daily, and Clarithromycin 500 mg Twice Daily for 7 or 10 Days):
| Days of RAC Therapy | Clarithromycin Pretreatment Results | Total Number | H. pylori Negative (Eradicated) | H. pylori Positive (Persistent) Post-Treatment Susceptibility Results | |||
|---|---|---|---|---|---|---|---|
| Sb | Ib | Rb | No MIC | ||||
| 7 | Susceptibleb | 129 | 103 | 2 | 0 | 1 | 23 |
| 7 | Intermediateb | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | Resistantb | 16 | 5 | 2 | 1 | 4 | 4 |
| 10 | Susceptibleb | 133 | 111 | 3 | 1 | 2 | 16 |
| 10 | Intermediateb | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | Resistantb | 9 | 1 | 0 | 0 | 5 | 3 |
a Includes only patients with pretreatment and post-treatment clarithromycin susceptibility test results.
b Susceptible (S) MIC ≤0.25 mcg/mL, Intermediate (I) MIC = 0.5 mcg/mL, Resistant ® MIC ≥1 mcg/mL
Patients with persistent H. pylori infection following rabeprazole, amoxicillin, and clarithromycin therapy will likely have clarithromycin resistant clinical isolates. Therefore, clarithromycin susceptibility testing should be done when possible. If resistance to clarithromycin is demonstrated or susceptibility testing is not possible, alternative antimicrobial therapy should be instituted.
Amoxicillin Susceptibility Test Results and Clinical/Bacteriological Outcomes
In the U.S. multicenter study, greater than 99% (558/560) of patients had H. pylori isolates which were considered to be susceptible (MIC ≤0.25 mcg/mL) to amoxicillin at baseline. The other 2 patients had baseline H. pylori isolates with an amoxicillin MIC of 0.5 mcg/mL, and both isolates were clarithromycin-resistant at baseline; in one case the H. pylori was eradicated. In the 7- and 10-day treatment groups 75% (107/145) and 79% (112/142), respectively, of the patients who had pretreatment amoxicillin susceptible MICs (≤0.25 mcg/mL) were eradicated of H. pylori. No patients developed amoxicillin-resistant H. pylori during therapy.
12.5. Pharmacogenomics
In a clinical study in evaluating ACIPHEX delayed-release tablets in Japanese adult patients categorized by CYP2C19 genotype (n=6 per genotype category), gastric acid suppression was higher in poor metabolizers as compared to extensive metabolizers. This could be due to higher rabeprazole plasma levels in poor metabolizers. The clinical relevance of this is not known. Whether or not interactions of rabeprazole sodium with other drugs metabolized by CYP2C19 would be different between extensive metabolizers and poor metabolizers has not been studied.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
In an 88/104-week carcinogenicity study in CD-1 mice, rabeprazole at oral doses up to 100 mg/kg/day did not produce any increased tumor occurrence. The highest tested dose produced a systemic exposure to rabeprazole (AUC) of 1.40 µg•hr/mL which is 1.6 times the human exposure (plasma AUC0-∞ = 0.88 µg•hr/mL) at the recommended dose for GERD (20 mg/day). In a 28-week carcinogenicity study in p53+/- transgenic mice, rabeprazole at oral doses of 20, 60, and 200 mg/kg/day did not cause an increase in the incidence rates of tumors but produced gastric mucosal hyperplasia at all doses. The systemic exposure to rabeprazole at 200 mg/kg/day is about 17 to 24 times the human exposure at the recommended dose for GERD. In a 104-week carcinogenicity study in Sprague-Dawley rats, males were treated with oral doses of 5, 15, 30 and 60 mg/kg/day and females with 5, 15, 30, 60, and 120 mg/kg/day. Rabeprazole produced gastric enterochromaffin-like (ECL) cell hyperplasia in male and female rats and ECL cell carcinoid tumors in female rats at all doses including the lowest tested dose. The lowest dose (5 mg/kg/day) produced a systemic exposure to rabeprazole (AUC) of about 0.1 µg•hr/mL which is about 0.1 times the human exposure at the recommended dose for GERD. In male rats, no treatment related tumors were observed at doses up to 60 mg/kg/day producing a rabeprazole plasma exposure (AUC) of about 0.2 µg•hr/mL (0.2 times the human exposure at the recommended dose for GERD).
Rabeprazole was positive in the Ames test, the Chinese hamster ovary cell (CHO/HGPRT) forward gene mutation test, and the mouse lymphoma cell (L5178Y/TK+/–) forward gene mutation test. Its demethylated-metabolite was also positive in the Ames test. Rabeprazole was negative in the in vitro Chinese hamster lung cell chromosome aberration test, the in vivo mouse micronucleus test, and the in vivo and ex vivo rat hepatocyte unscheduled DNA synthesis (UDS) tests.
Rabeprazole at intravenous doses up to 30 mg/kg/day (plasma AUC of 8.8 µg•hr/mL, about 10 times the human exposure at the recommended dose for GERD) was found to have no effect on fertility and reproductive performance of male and female rats.
14. Clinical Studies
14.1 Healing of Erosive or Ulcerative GERD in Adults
In a U.S., multicenter, randomized, double-blind, placebo-controlled study, 103 patients were treated for up to eight weeks with placebo, 10 mg, 20 mg, or 40 mg ACIPHEX delayed-release tablets once daily. For this and all studies of GERD healing, only patients with GERD symptoms and at least grade 2 esophagitis (modified Hetzel-Dent grading scale) were eligible for entry. Endoscopic healing was defined as grade 0 or 1. Each rabeprazole dose was significantly superior to placebo in producing endoscopic healing after four and eight weeks of treatment. The percentage of patients demonstrating endoscopic healing was as follows:
Table 7. Healing of Erosive or Ulcerative Gastroesophageal Reflux Disease (GERD) Percentage of Patients Healed:
| Week | ACIPHEX delayed-release tablets | Placebo N=25 | ||
|---|---|---|---|---|
| 10 mg once daily N=27 | 20 mg once daily N=25 | 40 mg once daily N=26 | ||
| 4 | 63%* | 56%* | 54%* | 0% |
| 8 | 93%* | 84%* | 85%* | 12% |
* (p<0.001 versus placebo)
In addition, there was a statistically significant difference in favor of the ACIPHEX 10 mg, 20 mg, and 40 mg doses compared to placebo at Weeks 4 and 8 regarding complete resolution of GERD heartburn frequency (p≤0.026). All ACIPHEX groups reported significantly greater rates of complete resolution of GERD daytime heartburn severity compared to placebo at Weeks 4 and 8 (p≤0.036). Mean reductions from baseline in daily antacid dose were statistically significant for all ACIPHEX groups when compared to placebo at both Weeks 4 and 8 (p≤0.007).
In a North American multicenter, randomized, double-blind, active-controlled study of 336 patients, the percentage of patients healed at endoscopy after four and eight weeks of treatment was statistically superior in the patients treated with ACIPHEX delayed-release tablets compared to ranitidine:
Table 8. Healing of Erosive or Ulcerative Gastroesophageal Reflux Disease (GERD) Percentage of Patients Healed:
| Week | 20 mg ACIPHEX delayed-release tablets once daily N=167 | Ranitidine 150 mg four times daily N=169 |
|---|---|---|
| 4 | 59%* | 36% |
| 8 | 87%* | 66% |
* (p<0.001 versus ranitidine)
A dose of 20 mg once daily of ACIPHEX delayed-release tablets was significantly more effective than ranitidine 150 mg four times daily in the percentage of patients with complete resolution of heartburn at Weeks 4 and 8 (p<0.001). ACIPHEX was also more effective in complete resolution of daytime heartburn (p≤0.025), and nighttime heartburn (p≤0.012) at both Weeks 4 and 8, with significant differences by the end of the first week of the study.
The recommended dosage of ACIPHEX delayed-release tablets is 20 mg once daily for 4 to 8 weeks.
14.2 Long-Term Maintenance of Healing of Erosive or Ulcerative GERD in Adults
The long-term maintenance of healing in patients with erosive or ulcerative GERD previously healed with gastric antisecretory therapy was assessed in two U.S., multicenter, randomized, double-blind, placebo-controlled studies of identical design of 52 weeks duration. The two studies randomized 209 and 285 patients, respectively, to receive either 10 mg or 20 mg of ACIPHEX delayed-release tablets once daily or placebo. As demonstrated in Tables 10 and 11 below, patients treated with ACIPHEX delayed-release tablets were significantly superior to placebo in both studies with respect to the maintenance of healing of GERD and the proportions of patients remaining free of heartburn symptoms at 52 weeks. The recommended dosage of ACIPHEX delayed-release tablets is 20 mg once daily.
Table 9. Percent of Patients in Endoscopic Remission:
| ACIPHEX delayed-release tablets | Placebo | ||
|---|---|---|---|
| 10 mg once daily | 20 mg once daily | ||
| Study 1 | N=66 | N=67 | N=70 |
| Week 4 | 83%* | 96%* | 44% |
| Week 13 | 79%* | 93%* | 39% |
| Week 26 | 77%* | 93%* | 31% |
| Week 39 | 76%* | 91%* | 30% |
| Week 52 | 73%* | 90%* | 29% |
| Study 2 | N=93 | N=93 | N=99 |
| Week 4 | 89%* | 94%* | 40% |
| Week 13 | 86%* | 91%* | 33% |
| Week 26 | 85%* | 89%* | 30% |
| Week 39 | 84%* | 88%* | 29% |
| Week 52 | 77%* | 86%* | 29% |
| COMBINED STUDIES | N=159 | N=160 | N=169 |
| Week 4 | 87%* | 94%* | 42% |
| Week 13 | 83%* | 92%* | 36% |
| Week 26 | 82%* | 91%* | 31% |
| Week 39 | 81%* | 89%* | 30% |
| Week 52 | 75%* | 87%* | 29% |
* (p<0.001 versus placebo)
Table 10. Percent of Patients Without Relapse in Heartburn Frequency and Daytime and Nighttime Heartburn Severity at Week 52:
| ACIPHEX delayed-release tablets | Placebo | ||
|---|---|---|---|
| 10 mg once daily | 20 mg once daily | ||
| Heartburn Frequency | |||
| Study 1 | 46/55 (84%)* | 48/52 (92%)* | 17/45 (38%) |
| Study 2 | 50/72 (69%)* | 57/72 (79%)* | 22/79 (28%) |
| Daytime Heartburn Severity | |||
| Study 1 | 61/64 (95%)* | 60/62 (97%)* | 42/61 (69%) |
| Study 2 | 73/84 (87%)† | 82/87 (94%)* | 67/90 (74%) |
| Nighttime Heartburn Severity | |||
| Study 1 | 57/61 (93%)* | 60/61 (98%)* | 37/56 (66%) |
| Study 2 | 67/80 (84%) | 79/87 (91%)† | 64/87 (74%) |
* p≤0.001 versus placebo
† 0.001<p<0.05 versus placebo
14.3 Treatment of Symptomatic GERD in Adults
Two U.S., multicenter, double-blind, placebo controlled studies were conducted in 316 adult patients with daytime and nighttime heartburn. Patients reported 5 or more periods of moderate to very severe heartburn during the placebo treatment phase the week prior to randomization. Patients were confirmed by endoscopy to have no esophageal erosions.
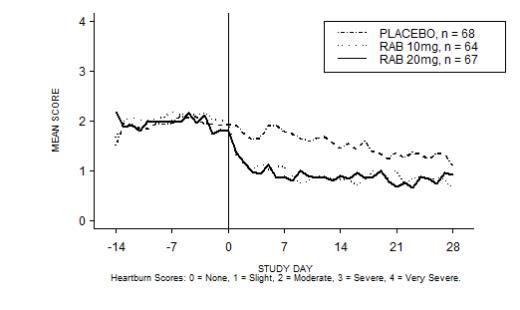
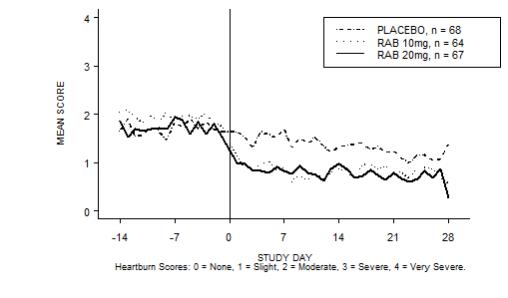
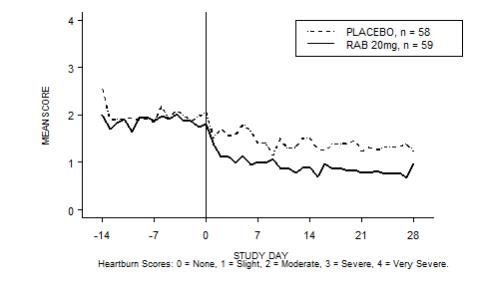
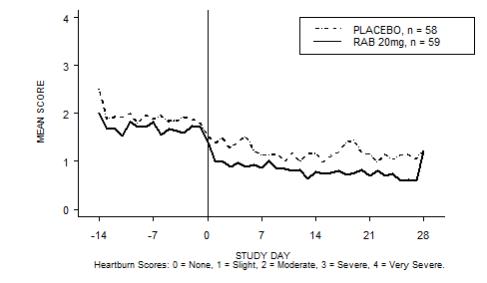
The percentage of heartburn free daytime and/or nighttime periods was greater with 20 mg ACIPHEX delayed-release tablets compared to placebo over the 4 weeks of study in Study RAB-USA-2 (47% vs. 23%) and Study RAB-USA-3 (52% vs. 28%). The mean decreases from baseline in average daytime and nighttime heartburn scores were significantly greater for ACIPHEX 20 mg as compared to placebo at week 4. Graphical displays depicting the daily mean daytime and nighttime scores are provided in Figures 2 to 5.
Figure 2. Mean Daytime Heartburn Scores RAB-USA-2:
Figure 3. Mean Nig httime Heartburn Scores RAB-USA-2:
Figure 4. Mean Daytime Heartburn Scores RAB-USA-3:
Figure 5. Mean Nig httime Heartburn Scores RAB-USA-3:
In addition, the combined analysis of these two studies showed 20 mg of ACIPHEX delayed-release tablets significantly improved other GERD-associated symptoms (regurgitation, belching, and early satiety) by week 4 compared with placebo (all p values <0.005).
A dose of 20 mg ACIPHEX delayed-release tablets also significantly reduced daily antacid consumption versus placebo over 4 weeks (p<0.001).
The recommended dosage of ACIPHEX delayed-release tablets is 20 mg once daily for 4 weeks.
14.4 Healing of Duodenal Ulcers in Adults
In a U.S., randomized, double-blind, multicenter study assessing the effectiveness of 20 mg and 40 mg of ACIPHEX delayed-release tablets once daily versus placebo for healing endoscopically defined duodenal ulcers, 100 patients were treated for up to four weeks. ACIPHEX was significantly superior to placebo in producing healing of duodenal ulcers. The percentages of patients with endoscopic healing are presented below:
Table 11. Healing of Duodenal Ulcers Percentage of Patients Healed:
| Week | ACIPHEX delayed-release tablets | Placebo N=33 | |
|---|---|---|---|
| 20 mg once daily N=34 | 40 mg once daily N=33 | ||
| 2 | 44% | 42% | 21% |
| 4 | 79%* | 91%* | 39% |
* p≤0.001 versus placebo
At Weeks 2 and 4, significantly more patients in the ACIPHEX 20 and 40 mg groups reported complete resolution of ulcer pain frequency (p≤0.018), daytime pain severity (p≤0.023), and nighttime pain severity (p≤0.035) compared with placebo patients. The only exception was the 40 mg group versus placebo at Week 2 for duodenal ulcer pain frequency (p=0.094). Significant differences in resolution of daytime and nighttime pain were noted in both ACIPHEX groups relative to placebo by the end of the first week of the study. Significant reductions in daily antacid use were also noted in both ACIPHEX groups compared to placebo at Weeks 2 and 4 (p<0.001).
An international randomized, double-blind, active-controlled trial was conducted in 205 patients comparing 20 mg ACIPHEX delayed-release tablets once daily with 20 mg omeprazole once daily. The study was designed to provide at least 80% power to exclude a difference of at least 10% between ACIPHEX and omeprazole, assuming four-week healing response rates of 93% for both groups. In patients with endoscopically defined duodenal ulcers treated for up to four weeks, ACIPHEX was comparable to omeprazole in producing healing of duodenal ulcers. The percentages of patients with endoscopic healing at two and four weeks are presented below:
Table 12. Healing of Duodenal Ulcers Percentage of Patients Healed:
| Week | ACIPHEX delayed-release tablets 20 mg once daily N=102 | Omeprazole 20 mg once daily N=103 | 95% Confidence Interval for the Treatment Difference (ACIPHEX – Omeprazole) |
|---|---|---|---|
| 2 | 69% | 61% | (–6%, 22%) |
| 4 | 98% | 93% | (–3%, 15%) |
ACIPHEX and omeprazole were comparable in providing complete resolution of symptoms.
The recommended dosage of ACIPHEX delayed-release tablets is 20 mg once daily for 4 weeks.
14.5 Helicobacter pylori Eradication in Patien ts with Peptic Ulcer Disease or Symptomatic Non-Ulcer Disease in Adults
The U.S. multicenter study was a double-blind, parallel-group comparison of ACIPHEX delayed-release tablets, amoxicillin, and clarithromycin for 3, 7, or 10 days vs. omeprazole, amoxicillin, and clarithromycin for 10 days. Therapy consisted of rabeprazole 20 mg twice daily, amoxicillin 1000 mg twice daily, and clarithromycin 500 mg twice daily (RAC) or omeprazole 20 mg twice daily, amoxicillin 1000 mg twice daily, and clarithromycin 500 mg twice daily (OAC). Patients with H. pylori infection were stratified in a 1:1 ratio for those with peptic ulcer disease (active or a history of ulcer in the past five years) [PUD] and those who were symptomatic but without peptic ulcer disease [NPUD], as determined by upper gastrointestinal endoscopy. The overall H. pylori eradication rates, defined as negative 13C-UBT for H. pylori ≥6 weeks from the end of the treatment are shown in the following table. The eradication rates in the 7-day and 10-day RAC regimens were found to be similar to 10-day OAC regimen using either the Intent-to-Treat (ITT) or Per-Protocol (PP) populations. Eradication rates in the RAC 3-day regimen were inferior to the other regimens.
Table 13. Helicobacter pylori Eradication at ≥6 Weeks After the End of Treatment:
| Treatment Group Percent (%) of Patients Cured (Number of Patients) | Difference (RAC – OAC) [95% Confidence Interval] | ||
|---|---|---|---|
| 7-day RAC* | 10-day OAC | ||
| Per Protocola | 84.3% (N=166) | 81.6% (N=179) | 2.8 [-5.2, 10.7] |
| Intent-to-Treatb | 77.3% (N=194) | 73.3% (N=206) | 4.0 [-4.4, 12.5] |
| 10-day RAC* | 10-day OAC | ||
| Per Protocola | 86.0% (N=171) | 81.6% (N=179) | 4.4 [-3.3, 12.1] |
| Intent-to-Treatb | 78.1% (N=196) | 73.3% (N=206) | 4.8 [-3.6, 13.2] |
| 3-day RAC | 10-day OAC | ||
| Per Protocola | 29.9% (N=167) | 81.6% (N=179) | -51.6 [-60.6, -42.6] |
| Intent-to-Treatb | 27.3% (N=187) | 73.3% (N=206) | -46.0 [-54.8, -37.2] |
a Patients were included in the analysis if they had H. pylori infection documented at baseline, defined as a positive 13C-UBT plus rapid urease test or culture and were not protocol violators. Patients who dropped out of the study due to an adverse event related to the study drug were included in the evaluable analysis as failures of therapy.
b Patients were included in the analysis if they had documented H. pylori infection at baseline as defined above and took at least one dose of study medication. All dropouts were included as failures of therapy.
* The 95% confidence intervals for the difference in eradication rates for 7-day RAC minus 10-day RAC are (-9.3, 6.0) in the PP population and (-9.0, 7.5) in the ITT population.
The recommended dosage of ACIPHEX delayed-release tablets is 20 mg twice daily with amoxicillin and clarithromycin for 7 days.
14.6 Pathological Hypersecretory Conditions, Including Zollinger-Ellison Syndrome in Adults
Twelve patients with idiopathic gastric hypersecretion or Zollinger-Ellison syndrome have been treated successfully with ACIPHEX delayed-release tablets at doses from 20 to 120 mg for up to 12 months. ACIPHEX produced satisfactory inhibition of gastric acid secretion in all patients and complete resolution of signs and symptoms of acid-peptic disease where present. ACIPHEX also prevented recurrence of gastric hypersecretion and manifestations of acid-peptic disease in all patients. The high doses of ACIPHEX used to treat this small cohort of patients with gastric hypersecretion were well tolerated.
The recommended starting dosage of ACIPHEX delayed-release tablets is 60 mg once daily.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.