BEYONTTRA Film-coated tablet Ref.[114796] Active ingredients: Acoramidis
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: BridgeBio Europe B.V., Weerdestein 97, Amsterdam, 1083 GG, The Netherlands
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Cardiac therapy, Other cardiac preparations
ATC code: C01EB25
Mechanism of action
Transthyretin amyloid cardiomyopathy is initiated by the dissociation of the transthyretin (TTR) tetramer into its constituent monomers. These misfold and aggregate as oligomeric amyloid precursors that deposit in the heart where they assemble into amyloid fibrils.
Acoramidis is a specific stabiliser of TTR. Acoramidis was designed to mimic the disease protective genetic variant (T119M), through the formation of hydrogen bonds with adjacent serine residues within both thyroxine binding sites of the tetramer. This interaction enhances the stability of the tetramer, inhibiting its dissociation into monomers, thus slowing the amyloidogenic process that results in ATTR-CM.
Pharmacodynamic effects
Near-complete transthyretin stabilisation was observed with acoramidis in wild-type and in all amyloidogenic variant genotypes tested, including the most prevalent genotypes V30M (p.V50M), T60A (p.T80A), and V122I (p.V142I). In the ATTRibute-CM study, in patients (wild-type and variant ATTR) treated with acoramidis (712 mg twice daily), near-complete (≥90%) TTR stabilisation was observed at the first post-dose initiation assessment (Day 28) and sustained through Month 30. For all post-baseline measurements (from Day 28 through Month 30), the TTR level was higher in the acoramidis group compared with placebo (at Month 30, mean change from baseline 9.1 mg/dL with acoramidis versus 1.3 mg/dL with placebo).
In ATTRibute-CM, the increase in N-terminal prohormone of brain natriuretic peptide (NT-proBNP) at Month 30 favoured acoramidis and was about half the increase seen with placebo. A lower increase in troponin I was also observed with acoramidis versus placebo.
In ATTRibute-CM, the mean serum creatinine (and estimated GFR) at baseline was 110.0 μmol/L (eGFR: 60.9 mL/min/1.73 m²) in the acoramidis group and 109.0 μmol/L (eGFR: 61.0 mL/min/1.73 m²) in the placebo group. At Day 28, there was a change from baseline in the mean serum creatinine (eGFR) that was greater in the acoramidis group (observed values on Day 28 serum creatinine: 129.3 μmol/L, eGFR: 52.4 mL/min/1.73 m²) compared with the placebo group (observed values on Day 28 serum creatinine: 110.6 μmol/L, eGFR: 60.0 mL/min/1.73 m²). After Day 28, serum creatinine (eGFR) remained stable in the acoramidis group for the remainder of the study. There was a progressive rise in serum creatinine, and corresponding progressive decrease in eGFR, in the placebo group from baseline through Month 30. At Month 30, serum creatinine was 123.4 μmol/L (eGFR: 55.1 mL/min/1.73 m²) and 117.2 μmol/L (eGFR: 57.2 mL/min/1.73 m²) for acoramidis and placebo respectively. The observed increase in serum creatinine, and corresponding decrease in eGFR, observed in acoramidis treated patients was reversible in the event of an interruption of therapy.
Cardiac electrophysiology
The maximum dose of acoramidis, 1 780 mg, studied as a single dose in healthy adult volunteers did not have a clinically relevant effect on cardiac conduction or repolarisation (no concentration-QTc effect was observed). These observations indicate a low risk of pro-arrhythmia.
Clinical efficacy
ATTRibute-CM was a multicentre, international, randomised, double-blind, placebo-controlled clinical study conducted in 632 participants with wild-type or variant (hereditary or de novo) ATTR-CM and heart failure NYHA Class I-III, with current or prior symptoms of heart failure. Participants were randomised in a 2:1 ratio to receive acoramidis 712 mg (n=421), or matching placebo (n=211) twice daily for 30 months. Treatment assignment was stratified by whether participants had variant ATTR-CM (ATTRv-CM) or wild-type ATTR-CM (ATTRwt-CM) and baseline disease severity, i.e., NT-proBNP level and renal function as defined by eGFR. Patients with eGFR <15 mL/min/1.73 m² were excluded from participation in the study.
Table 2. Demographics and baseline characteristics (mITT population1):
| Characteristic | Acoramidis N=409 | Placebo N=202 |
|---|---|---|
| Age — years | ||
| Mean (standard deviation) | 77.3 (6.5) | 77.0 (6.7) |
| Sex — number (%) | ||
| Male | 374 (91.4) | 181 (89.6) |
| Female | 35 (8.6) | 21 (10.4) |
| TTR genotype2 — number (%) | ||
| ATTRv | 39 (9.5) | 20 (9.9) |
| ATTRwt | 370 (90.5) | 182 (90.1) |
| NYHA class — number (%) | ||
| NYHA class I | 51 (12.5) | 17 (8.4) |
| NYHA class II | 288 (70.4) | 156 (77.2) |
| NYHA class III | 70 (17.1) | 29 (14.4) |
| eGFR2 (mL/min/1.73 m²) – number (%) | ||
| eGFR ≥45 | 344 (84.1) | 173 (85.6) |
| eGFR <45 | 65 (15.9) | 29 (14.4) |
| NT-proBNP2 (pg/mL) – number (%) | ||
| ≤3 000 | 268 (65.5) | 133 (65.8) |
| >3 000 | 141 (34.5) | 69 (34.2) |
| ATTR NAC stage3 — number (%) | ||
| I | 241 (58.9) | 120 (59.4) |
| II | 130 (31.8) | 66 (32.7) |
| III | 38 (9.3) | 16 (7.9) |
| History of permanent pacemaker – number (%) | 77 (18.8) | 38 (18.8) |
| History of atrial fibrillation – number (%) | 236 (57.7) | 117 (57.9) |
Abbreviations: ATTRv = variant transthyretin amyloid, ATTRwt = wild-type transthyretin amyloid, NAC = National Amyloidosis Centre (London UK), NYHA = New York Heart Association, eGFR = estimated glomerular filtration rate, NT-proBNP = N-terminal prohormone of brain natriuretic peptide, TTR = transthyretin
1 mITT = modified intent to treat (baseline eGFR ≥30 mL/min/1.73 m²).
2 Stratification factors.
3 NAC Stage I (NT-proBNP ≤3 000 pg/mL and eGFR ≥45 mL/min/1.73 m²), Stage II (NT-proBNP ≤3 000 pg/mL and eGFR <45 mL/min/1.73 m² or NT-proBNP >3 000 pg/mL and eGFR ≥45 mL/min/1.73 m²), Stage III (NT-proBNP >3 000 pg/mL and eGFR <45 mL/min/1.73 m²).
Participants were permitted to initiate open label tafamidis if prescribed as a concomitant medicinal product after 12 months in the study. A total of 107 participants received tafamidis, 61 (14.9%) in the acoramidis arm and 46 (22.8%) in the placebo arm.
The primary objective of the study was to establish superiority of acoramidis versus placebo on a hierarchical endpoint that included all-cause mortality (ACM) and cumulative frequency of cardiovascular-related hospitalisation (CVH). Secondary objectives included assessment of ACM, CVH, 6-minute walk distance, Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score (a measure of quality of life), serum TTR level and NT-proBNP. The main efficacy analyses were conducted in the 611 participants in the modified intent to treat (mITT) population without any adjustment for the introduction of open label tafamidis.
Efficacy analysis
The efficacy analysis applied the stratified Finkelstein-Schoenfeld (F-S) test hierarchically to ACM and CVH over the 30-month study. The method compared each participant to every other participant within each stratum in a pair-wise manner. In this hierarchical approach, participants in each pair are first compared on ACM, and then on CVH only if the comparison on ACM resulted in a tie. The result of this analysis was statistically significant (Table 3).
All-cause mortality was reported in 19.3% and 25.7% of participants in the acoramidis and placebo groups, respectively. The majority (79%) of deaths were cardiovascular (CV)-related with acoramidis demonstrating a 30% relative risk reduction in CV-related mortality compared with placebo. CV-related mortality was reported in 14.9% and 21.3% of participants in the acoramidis and placebo groups, respectively; hazard ratio: 0.709 (95% CI: 0.476, 1.054, p=0.0889, Cox proportional hazards model).
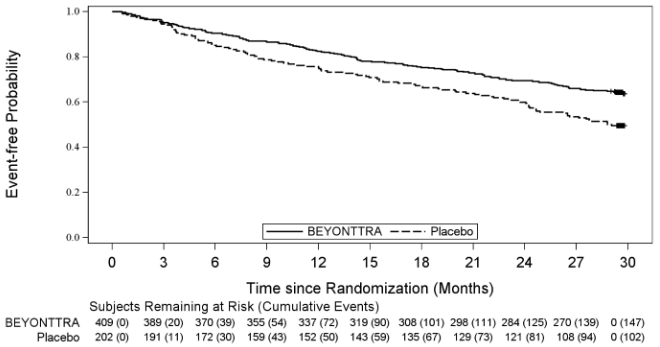
A Cox regression analysis indicated a 35.5% decrease in the risk of the composite of ACM or first CV hospitalisation (hazard ratio: 0.645 [95% CI: 0.500, 0.832; p=0.0008]). Separation in the Kaplan- Meier curves was observed at Month 3 and steadily diverged through Month 30 (Figure 1).
The efficacy results on ACM and CVH demonstrated in the mITT population were also observed in the ITT population (all randomised subjects irrespective of baseline eGFR).
Table 3. Efficacy results on Finkelstein-Schoenfeld analysis, all-cause mortality and cardiovascular-related hospitalisation at Month 30 in ATTRibute-CM (mITT population):
| Parameter | Acoramidis N=409 | Placebo N=202 |
|---|---|---|
| Combination of ACM and cumulative frequency of CVH Win ratio (95% CI) F-S1 p-value | 1.464 (1.067, 2.009) p=0.0182 | |
| Number (%) of participants alive at Month 302 | 330 (80.7%) | 150 (74.3%) |
| Number (%) of participants with CVH Number of total CVH events Frequency of CVH per year per participant (mean)3 | 109 (26.7%) 182 0.29 | 86 (42.6%) 170 0.55 |
| Relative risk ratio4 p-value | 0.496 p<0.0001 | |
Abbreviations: F-S = Finkelstein-Schoenfeld; ACM = all-cause mortality; CVH = cardiovascular hospitalisation; mITT = modified intent-to-treat; CI = confidence interval
1 The F-S method compares every participant pair within each stratum in a hierarchical fashion, starting with ACM. If pairs are tied on ACM, they are then assessed on CVH.
2 Heart transplantation and cardiac mechanical assist device implantation are considered indicators of approaching end stage. As such, these events are treated in the analysis as equivalent to death. Therefore, such participants are not included in the count of "Number of participants alive at Month 30" even if such participants are alive based on 30-month vital status follow-up assessment. Vital status at Month 30 was known for all participants.
3 CVH per year for each participant is calculated as (participant's total number of observed CVH) / (duration of follow-up in years) and include events of clinical interest (EOCI). EOCI is defined as medical visits (e.g., emergency department/ward, urgent care clinic, day clinic) of <24 hours for intravenous diuretic therapy for management of decompensated heart failure.
4 From negative binomial regression model.
Figure 1. Time to all-cause mortality or first cardiovascular-related hospitalisation:
6-Minute Walk Distance (6MWD) and KCCQ
The treatment effect of acoramidis on functional capacity and health status was assessed by the 6MWD and the KCCQ Overall Summary score (KCCQ-OS); composed of the physical limitation, symptom, social limitation and quality of life domains), respectively (Table 4). A treatment effect favouring acoramidis was first observed for 6MWD and KCCQ-OS at Month 18 and Month 3, respectively, and was sustained through Month 30.
Table 4. 6MWD and KCCQ-OS scores:
| Endpoints* | Baseline Mean (SD) | Change from Baseline to Month 30, LS Mean (SE) | Treatment Difference from Placebo LS Mean (96% CI) | p-value | ||
|---|---|---|---|---|---|---|
| Acoramidis N=409 | Placebo N=202 | Acoramidis N=409 | Placebo N=202 | |||
| 6MWD (metres) | 362.78 (103.50) | 351.51 (93.83) | -64.65 (5.51) | -104.29 (7.77) | 39.64 (20.18, 59.10) | <0.0001 |
| KCCQ-OS | 71.73 (19.37) | 70.48 (20.65) | -11.48 (1.18) | -21.42 (1.65) | 9.94 (5.79, 14.10) | <0.0001 |
Abbreviations: 6MWD = 6-minute walk distance; CI = confidence interval; KCCQ-OS = Kansas City Cardiomyopathy Questionnaire Overall Summary score, LS = least squares, SD = standard deviation, SE = standard error
* Higher values indicate better health status.
Subgroup analysis
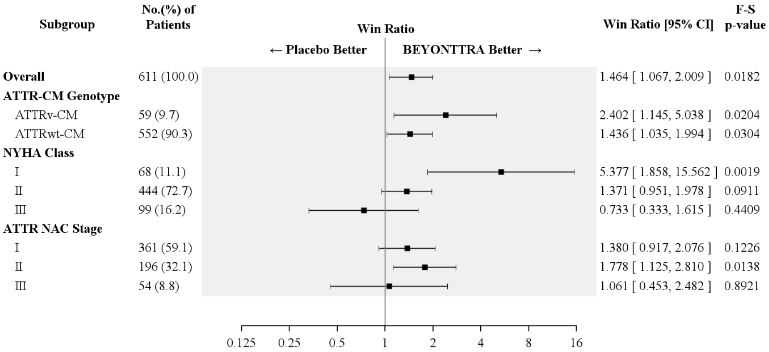
Results from the F-S test applied to ACM and CVH (complemented by the win ratio) consistently favoured acoramidis versus placebo across the stratification parameter (wild type or variant), NYHA class and ATTR National Amyloidosis Centre (NAC) stage subgroups (Figure 2).
Figure 2. Hierarchical combination of all-cause mortality and CV-related hospitalisation, Finkelstein-Schoenfeld and win ratio results overall and by subgroup (mITT population)1:
Abbreviations: ACM = all-cause mortality; ATTRwt-CM = wild-type ATTR-CM; ATTRv-CM = variant ATTR-CM; CVH = cardiovascular-related hospitalisation; F-S = Finkelstein-Schoenfeld; NAC = National Amyloidosis Centre (London, UK); NYHA = New York Heart Association; NAC Stage I (NT-proBNP ≤3 000 pg/mL and eGFR ≥45 mL/min/1.73 m²), Stage II (NT-proBNP ≤3 000 pg/mL and eGFR <45 mL/min/1.73 m² or NT-proBNP >3 000 pg/mL and eGFR ≥45 mL/min/1.73 m²), Stage III (NT-proBNP >3 000 pg/mL and eGFR <45 mL/min/1.73 m²)
1 The win ratio is the number of pairs with acoramidis treated-participant "wins" divided by number of pairs with placebo-treated participant "wins."
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with BEYONTTRA in all subsets of the paediatric population in ATTR-CM (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Absorption
The increase in exposure parameters (area under the concentration-time curve [AUC] and maximum concentration [Cmax]) was less than dose-proportional over single (up to 1 780 mg) or multiple (up to 712 mg) twice daily dosing.
Following oral administration, acoramidis is rapidly absorbed and peak plasma concentration of unchanged acoramidis is usually achieved within 1 hour. Increases in plasma concentration were observed for acoramidis doses from 44.5 mg once daily to 712 mg once daily. Plasma exposures appeared to saturate at acoramidis doses over 712 mg to 1 068 mg. A steady state is achieved by 10 days of dosing with 712 mg twice daily, and repeated dosing results in minor (approximately 1.3- to 1.6-fold) accumulation of acoramidis.
The absolute bioavailability is not known; however at least 75-80% of orally administered single 712 mg dose is absorbed based on a human ADME (absorption, distribution, metabolism, excretion) study.
The overall extent of absorption of acoramidis is not influenced by food intake.
Distribution
The apparent steady state volume of distribution of 712 mg acoramidis dosed twice daily is 654 litres. In vitro binding of acoramidis to human plasma proteins is 96.4%. Acoramidis primarily binds to TTR.
Biotransformation
The metabolism of acoramidis was characterised following the administration of a single oral dose of [14C]-acoramidis to healthy adult volunteers. Acoramidis is metabolised predominantly by glucuronidation, with acoramidis-β-D-glucuronide (acoramidis-AG) being the predominant metabolite (7.6% of total circulating radioactivity). Acoramidis-AG is approximately 3-fold less pharmacologically active than acoramidis, has a low potential for covalent binding, and does not meaningfully contribute to pharmacological activity.
Elimination and excretion
The terminal half-life of acoramidis is approximately 27 hours after a single dose. At steady state, the apparent oral clearance of acoramidis is 15.6 L/h.
After administration of a single oral dose of [14C]-acoramidis to healthy adult volunteers, approximately 34% of dose radioactivity was recovered in faeces (acoramidis being the major component) and approximately 68% was recovered in urine. The percent of unchanged acoramidis in the urine was <10%.
Special populations
No clinically significant differences in the pharmacokinetics of acoramidis were observed based on age (18.0-89.3 years), race/ethnicity (including Japanese and non-Japanese), sex, or renal impairment (eGFR 25.4-157 mL/min/1.73 m²).
Based on population PK modelling, steady-state acoramidis AUC was 37% higher for healthy subjects than for the patient population. Also, relative to White subjects, steady-state AUC was 23% higher for Black subjects and 38% higher for non-White, non-Black subjects. These effects are within the range of inter-individual variability (CV=38%). The model also predicted lack of clinically significant differences in the pharmacokinetics of acoramidis due to body weight, within the body weights range of 50.9 to 133 kg.
A dedicated renal-impairment study was not conducted because acoramidis is not substantially eliminated by the renal route. However, despite the main metabolite (acoramidis-AG) having no clinically relevant contribution to pharmacological activity in the studied population, data in patients with severe renal impairment (creatinine clearance <30 mL/min) are limited and there are no data for patients on dialysis. Clearance of the acoramidis metabolite acoramidis-AG might be affected by severe renal impairment resulting potentially in higher systemic exposure of acoramidis-AG. While this potential increase in acoramidis-AG exposure is not expected to have a clinically meaningful contribution to pharmacologic activity, acoramidis should be used with caution in patients with severe renal impairment.
Acoramidis has not been studied in patients with hepatic impairment.
5.3. Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, developmental and reproductive toxicology (fertility and embryo-foetal development).
In the rat pre- and postnatal development study with acoramidis, decreased pup survival, reduced pup weights, and learning deficits were observed following maternal dose administration during pregnancy and lactation with acoramidis at 1 000 mg/kg/day. Severe maternal toxicity including mortalities and weight loss during the period of organogenesis was also observed at this dose. The no-observed-adverse-effect-level (NOAEL) in pre- and postnatal development toxicity study in rats were established at the tested dose of 350 mg/kg/day acoramidis, (AUC values were approximatively 21-fold the human exposure at the clinical dose of acoramidis).
Placental transfer and milk excretion studies in animals were not performed.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.