BUCCOLAM Oromucosal solution Ref.[7765] Active ingredients: Midazolam
Source: European Medicines Agency (EU) Revision Year: 2018 Publisher: Shire Services BVBA, rue Montoyer 47, 1000 Brussels, Belgium
Therapeutic indications
Treatment of prolonged, acute, convulsive seizures in infants, toddlers, children and adolescents (from 3 months to <18 years).
BUCCOLAM must only be used by parents/carers where the patient has been diagnosed to have epilepsy.
For infants between 3-6 months of age treatment should be in a hospital setting where monitoring is possible and resuscitation equipment is available. See section 4.2.
Posology and method of administration
Posology
Standard doses are indicated below:
| Age range | Dose | Label colour |
|---|---|---|
| 3 to 6 months hospital setting | 2.5 mg | Yellow |
| > 6 months to < 1 year | 2.5 mg | Yellow |
| 1 year to < 5 years | 5 mg | Blue |
| 5 years to < 10 years | 7.5 mg | Purple |
| 10 years to < 18 years | 10 mg | Orange |
Carers should only administer a single dose of midazolam. If the seizure has not stopped within 10 minutes after administration of midazolam, emergency medical assistance must be sought and the empty syringe given to the healthcare professional to provide information on the dose received by the patient.
A second or repeat dose when seizures re-occur after an initial response should not be given without prior medical advice (see section 5.2).
Special populations
Renal impairment
No dose adjustment is required, however, BUCCOLAM should be used with caution in patients with chronic renal failure as elimination of midazolam may be delayed and the effects prolonged. (see section 4.4)
Hepatic impairment
Hepatic impairment reduces the clearance of midazolam with a subsequent increase in terminal halflife. Therefore, the clinical effects may be stronger and prolonged, hence careful monitoring of the clinical effects and vital signs is recommended following administration of midazolam in patients with hepatic impairment (see section 4.4).
BUCCOLAM is contraindicated in patients with severe hepatic impairment (see section 4.3).
Paediatric population
The safety and efficacy of midazolam in children aged 0 to 3 months has not been established. No data are available.
Method of administration
BUCCOLAM is for oromucosal use. The full amount of solution should be inserted slowly into the space between the gum and the cheek. Laryngo-tracheal insertion should be avoided to prevent accidental aspiration of the solution. If necessary (for larger volumes and/or smaller patients), approximately half the dose should be given slowly into one side of the mouth, then the other half given slowly into the other side.
For detailed instructions on how to administer the medicinal product, see section 6.6.
Precautions to be taken before handling or administering the medicinal product
No needle, intravenous tubing or any other device for parenteral administration should be attached to the oral syringe.
BUCCOLAM is not for intravenous use.
The oral syringe cap should be removed before use to avoid risk of choking
Overdose
Symptoms
Midazolam overdose can present a threat to life if the patient has pre-existing respiratory or cardiac insufficiency, or when combined with other CNS depressants (including alcohol).
Overdose of benzodiazepines is usually manifested by degrees of central nervous system depression ranging from drowsiness to coma. In mild cases, symptoms include drowsiness, mental confusion and lethargy, in more serious cases, symptoms may include ataxia, hypotonia, hypotension, respiratory depression, rarely coma and very rarely death.
Management
In the management of overdose with any medicinal product, it should be borne in mind that multiple agents may have been taken.
Following overdose with oral midazolam, vomiting should be induced (within one hour) if the patient is conscious or gastric lavage undertaken with the airway protected if the patient is unconscious. If there is no advantage in emptying the stomach, activated charcoal should be given to reduce absorption. Special attention should be paid to respiratory and cardiovascular functions in intensive care.
Flumazenil may be useful as an antidote.
Shelf life
18 months.
Special precautions for storage
Keep the oral syringe in the protective plastic tube.
Do not refrigerate or freeze.
Nature and contents of container
Amber, pre-filled needle-free oral syringe (polypropylene) with plunger (polypropylene) and end cap (high density polyethylene) packed in a protective, capped plastic tube.
| Strength | Volume of solution | Syringe volume | Age range | Label colour |
|---|---|---|---|---|
| 2.5 mg | 0.5 ml | 1 ml | 3 months to < 1 year | Yellow |
| 5 mg | 1 ml | 3 ml | 1 year to < 5 years | Blue |
| 7.5 mg | 1.5 ml | 3 ml | 5 years to < 10 years | Purple |
| 10 mg | 2 ml | 3 ml | 10 years to < 18 years | Orange |
BUCCOLAM is available in cartons containing 4 pre-filled syringes.
Special precautions for disposal and other handling
Administration of BUCCOLAM
BUCCOLAM is not for intravenous use.
Step 1: Hold the plastic tube, break the seal at one end and pull the cap off. Take the syringe out of the tube.
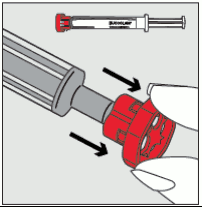
Step 2: Pull the red cap off the tip of the syringe and dispose of it safely. 12
Step 3: Using the finger and thumb gently pinch and pull back the child’s cheek. Put the tip of the syringe into the back of the space between the inside of the cheek and the lower gum.
Step 4: Slowly press the syringe plunger until the plunger stops. The full amount of solution should be inserted slowly into the space between the gum and the cheek (buccal cavity). If necessary (for larger volumes and/or smaller patients), approximately half the dose should be given slowly into one side of the mouth, then the other half given slowly into the other side.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.